ARIKAYCE targets MAC at the site of infection1-3
MAC can persist in biofilms or intracellularly within macrophages.4
Challenges to treating MAC4:
- Effective delivery and distribution of high amounts of antibiotics to the lungs
- Penetration of biofilms and macrophages to reach the sites where MAC lives and replicates
- Limit systemic exposure
ARIKAYCE was developed to penetrate biofilm and kill MAC1,3-5:

ARIKAYCE delivers1,4:
- Liposomal and free amikacin into the lungs through nebulization1
- ~4 times more amikacin into macrophages compared to cells exposed to the same concentrations of free amikacin based on in vitro data from a study of cultured human macrophages4
The clinical significance of these data is unknown.
In the 2018 CLSI guidelines, the MIC breakpoint for resistance is ≥128 μg/mL for ARIKAYCE6
An amikacin MIC level of ≤64 μg/mL is considered susceptible for ARIKAYCE.6
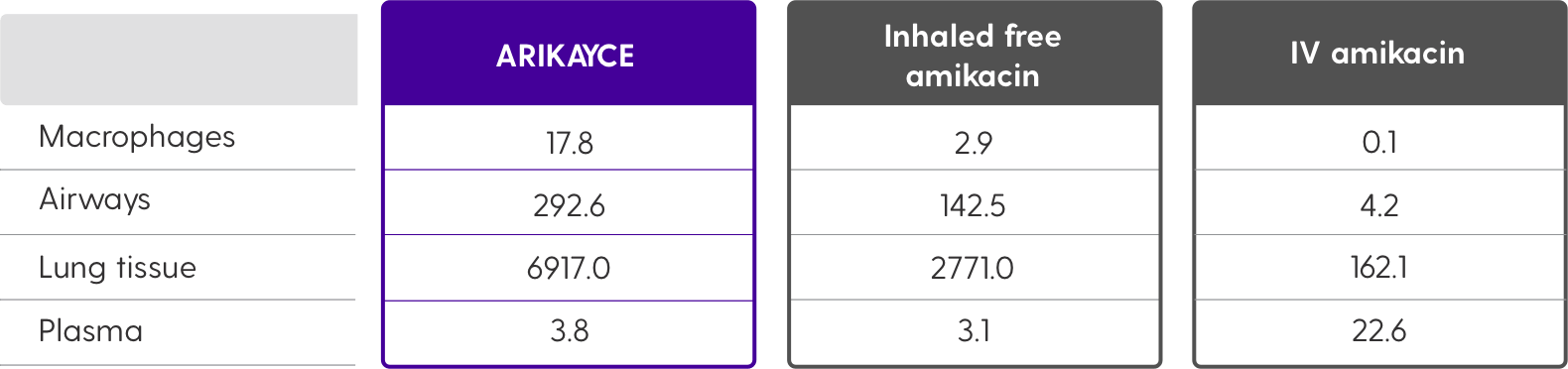
More uptake into macrophages with ARIKAYCE as shown in an in vivo study4
ARIKAYCE in vivo amikacin uptake into macrophages in rats compared to inhaled free and IV amikacin4
Values represent AUC calculated for each group using the mean concentrations at each time point. AUC2-24 was calculated for macrophage, airway, and plasma exposures, whereas AUC0-24 was calculated for lung exposure.4
Scroll to see full chart. →

| ARIKAYCE | Inhaled free amikacin | IV amikacin | |
|---|---|---|---|
| Macrophages | 17.8 | 2.9 | 0.1 |
| Airways | 292.6 | 142.5 | 4.2 |
| Lung tissue | 6917.0 | 2771.0 | 162.1 |
| Plasma | 3.8 | 3.1 | 22.6 |
The clinical relevance of this is unknown.
Limited systemic exposure with ARIKAYCE1
Following 3 months of once-daily inhalation of 590-mg ARIKAYCE in MAC patients, the mean serum AUC0-24 and Cmax were measured and compared with the approved dosage of 15 mg/kg IV amikacin once daily in healthy adults.
Amikacin serum levels were lower with ARIKAYCE vs IV amikacin1*
Scroll to see full chart. →


The clinical relevance of this is unknown.