The ARIKAYCE Academy
The ARIKAYCE Academy is an online resource center that provides access to insightful commentary, featuring experts in MAC lung disease. Watch videos at your convenience and download materials for later reading.

Explore in-depth information about the management of MAC lung disease, including insights from experts in the field
This content was created by Insmed. Experts have been compensated for their time.
See experts review data and share insights
The appropriate patient
Dr Julie Philley identifies which patients are appropriate for having ARIKAYCE added to their multidrug regimen.
ARIKAYCE should be used in the MAC lung disease patient who is not responding, or who's not responded, to a standard multidrug regimen for MAC. These are patients who have been on treatment for at least 6 months and remain culture-positive.
IMPORTANT SAFETY INFORMATION
WARNING: RISK OF INCREASED RESPIRATORY ADVERSE REACTIONS
ARIKAYCE has been associated with an increased risk of respiratory adverse reactions, including hypersensitivity pneumonitis, hemoptysis, bronchospasm, and exacerbation of underlying pulmonary disease that have led to hospitalizations in some cases.
Hypersensitivity Pneumonitis has been reported with the use of ARIKAYCE in the clinical trials. Hypersensitivity pneumonitis (reported as allergic alveolitis, pneumonitis, interstitial lung disease, allergic reaction to ARIKAYCE) was reported at a higher frequency in patients treated with ARIKAYCE plus background regimen (3.1%) compared to patients treated with a background regimen alone (0%). Most patients with hypersensitivity pneumonitis discontinued treatment with ARIKAYCE and received treatment with corticosteroids. If hypersensitivity pneumonitis occurs, discontinue ARIKAYCE and manage patients as medically appropriate.
Hemoptysis has been reported with the use of ARIKAYCE in the clinical trials. Hemoptysis was reported at a higher frequency in patients treated with ARIKAYCE plus background regimen (17.9%) compared to patients treated with a background regimen alone (12.5%). If hemoptysis occurs, manage patients as medically appropriate.
Bronchospasm has been reported with the use of ARIKAYCE in the clinical trials. Bronchospasm (reported as asthma, bronchial hyperreactivity, bronchospasm, dyspnea, dyspnea exertional, prolonged expiration, throat tightness, wheezing) was reported at a higher frequency in patients treated with ARIKAYCE plus background regimen (28.7%) compared to patients treated with a background regimen alone (10.7%). If bronchospasm occurs during the use of ARIKAYCE, treat patients as medically appropriate.
Exacerbations of underlying pulmonary disease has been reported with the use of ARIKAYCE in the clinical trials. Exacerbations of underlying pulmonary disease (reported as chronic obstructive pulmonary disease (COPD), infective exacerbation of COPD, infective exacerbation of bronchiectasis) have been reported at a higher frequency in patients treated with ARIKAYCE plus background regimen (14.8%) compared to patients treated with background regimen alone (9.8%). If exacerbations of underlying pulmonary disease occur during the use of ARIKAYCE, treat patients as medically appropriate.
Anaphylaxis and Hypersensitivity Reactions: Serious and potentially life-threatening hypersensitivity reactions, including anaphylaxis, have been reported in patients taking ARIKAYCE. Signs and symptoms include acute onset of skin and mucosal tissue hypersensitivity reactions (hives, itching, flushing, swollen lips/tongue/uvula), respiratory difficulty (shortness of breath, wheezing, stridor, cough), gastrointestinal symptoms (nausea, vomiting, diarrhea, crampy abdominal pain), and cardiovascular signs and symptoms of anaphylaxis (tachycardia, low blood pressure, syncope, incontinence, dizziness). Before therapy with ARIKAYCE is instituted, evaluate for previous hypersensitivity reactions to aminoglycosides. If anaphylaxis or a hypersensitivity reaction occurs, discontinue ARIKAYCE and institute appropriate supportive measures.
Ototoxicity has been reported with the use of ARIKAYCE in the clinical trials. Ototoxicity (including deafness, dizziness, presyncope, tinnitus, and vertigo) were reported with a higher frequency in patients treated with ARIKAYCE plus background regimen (17%) compared to patients treated with background regimen alone (9.8%). This was primarily driven by tinnitus (7.6% in ARIKAYCE plus background regimen vs 0.9% in the background regimen alone arm) and dizziness (6.3% in ARIKAYCE plus background regimen vs 2.7% in the background regimen alone arm). Closely monitor patients with known or suspected auditory or vestibular dysfunction during treatment with ARIKAYCE. If ototoxicity occurs, manage patients as medically appropriate, including potentially discontinuing ARIKAYCE.
Nephrotoxicity was observed during the clinical trials of ARIKAYCE in patients with MAC lung disease but not at a higher frequency than background regimen alone. Nephrotoxicity has been associated with the aminoglycosides. Close monitoring of patients with known or suspected renal dysfunction may be needed when prescribing ARIKAYCE.
Neuromuscular Blockade: Patients with neuromuscular disorders were not enrolled in ARIKAYCE clinical trials. Patients with known or suspected neuromuscular disorders, such as myasthenia gravis, should be closely monitored since aminoglycosides may aggravate muscle weakness by blocking the release of acetylcholine at neuromuscular junctions.
Embryo-Fetal Toxicity: Aminoglycosides can cause fetal harm when administered to a pregnant woman. Aminoglycosides, including ARIKAYCE, may be associated with total, irreversible, bilateral congenital deafness in pediatric patients exposed in utero. Patients who use ARIKAYCE during pregnancy, or become pregnant while taking ARIKAYCE should be apprised of the potential hazard to the fetus.
Contraindications: ARIKAYCE is contraindicated in patients with known hypersensitivity to any aminoglycoside.
Most Common Adverse Reactions: The most common adverse reactions in Trial 1 at an incidence ≥5% for patients using ARIKAYCE plus background regimen compared to patients treated with background regimen alone were dysphonia (47% vs 1%), cough (39% vs 17%), bronchospasm (29% vs 11%), hemoptysis (18% vs 13%), ototoxicity (17% vs 10%), upper airway irritation (17% vs 2%), musculoskeletal pain (17% vs 8%), fatigue and asthenia (16% vs 10%), exacerbation of underlying pulmonary disease (15% vs 10%), diarrhea (13% vs 5%), nausea (12% vs 4%), pneumonia (10% vs 8%), headache (10% vs 5%), pyrexia (7% vs 5%), vomiting (7% vs 4%), rash (6% vs 2%), decreased weight (6% vs 1%), change in sputum (5% vs 1%), and chest discomfort (5% vs 3%).
Drug Interactions: Avoid concomitant use of ARIKAYCE with medications associated with neurotoxicity, nephrotoxicity, and ototoxicity. Some diuretics can enhance aminoglycoside toxicity by altering aminoglycoside concentrations in serum and tissue. Avoid concomitant use of ARIKAYCE with ethacrynic acid, furosemide, urea, or intravenous mannitol.
Overdosage: Adverse reactions specifically associated with overdose of ARIKAYCE have not been identified. Acute toxicity should be treated with immediate withdrawal of ARIKAYCE, and baseline tests of renal function should be undertaken. Hemodialysis may be helpful in removing amikacin from the body. In all cases of suspected overdosage, physicians should contact the Regional Poison Control Center for information about effective treatment.
INDICATION
LIMITED POPULATION: ARIKAYCE® is indicated in adults, who have limited or no alternative treatment options, for the treatment of Mycobacterium avium complex (MAC) lung disease as part of a combination antibacterial drug regimen in patients who do not achieve negative sputum cultures after a minimum of 6 consecutive months of a multidrug background regimen therapy. As only limited clinical safety and effectiveness data for ARIKAYCE are currently available, reserve ARIKAYCE for use in adults who have limited or no alternative treatment options. This drug is indicated for use in a limited and specific population of patients.
This indication is approved under accelerated approval based on achieving sputum culture conversion (defined as 3 consecutive negative monthly sputum cultures) by Month 6. Clinical benefit has not yet been established. Continued approval for this indication may be contingent upon verification and description of clinical benefit in confirmatory trials.
Limitation of Use: ARIKAYCE has only been studied in patients with refractory MAC lung disease defined as patients who did not achieve negative sputum cultures after a minimum of 6 consecutive months of a multidrug background regimen therapy. The use of ARIKAYCE is not recommended for patients with non-refractory MAC lung disease.
Additional appropriate patients
Dr Wendi Drummond shares additional patient types who may also be appropriate for ARIKAYCE.
INDICATION
LIMITED POPULATION: ARIKAYCE® is indicated in adults, who have limited or no alternative treatment options, for the treatment of Mycobacterium avium complex (MAC) lung disease as part of a combination antibacterial drug regimen in patients who do not achieve negative sputum cultures after a minimum of 6 consecutive months of a multidrug background regimen therapy. As only limited clinical safety and effectiveness data for ARIKAYCE are currently available, reserve ARIKAYCE for use in adults who have limited or no alternative treatment options. This drug is indicated for use in a limited and specific population of patients.
This indication is approved under accelerated approval based on achieving sputum culture conversion (defined as 3 consecutive negative monthly sputum cultures) by Month 6. Clinical benefit has not yet been established. Continued approval for this indication may be contingent upon verification and description of clinical benefit in confirmatory trials.
Limitation of Use: ARIKAYCE has only been studied in patients with refractory MAC lung disease defined as patients who did not achieve negative sputum cultures after a minimum of 6 consecutive months of a multidrug background regimen therapy. The use of ARIKAYCE is not recommended for patients with non-refractory MAC lung disease.
Physicians may also consider reviewing their patient panels of existing patients who've been on guideline-based therapy for greater than 6 months, sometimes 9, 12 to 15 months, who may not have converted their sputum cultures to negative. There may be a number of these patients who would benefit from ARIKAYCE therapy in that clinical setting.
IMPORTANT SAFETY INFORMATION
WARNING: RISK OF INCREASED RESPIRATORY ADVERSE REACTIONS
ARIKAYCE has been associated with an increased risk of respiratory adverse reactions, including hypersensitivity pneumonitis, hemoptysis, bronchospasm, and exacerbation of underlying pulmonary disease that have led to hospitalizations in some cases.
Hypersensitivity Pneumonitis has been reported with the use of ARIKAYCE in the clinical trials. Hypersensitivity pneumonitis (reported as allergic alveolitis, pneumonitis, interstitial lung disease, allergic reaction to ARIKAYCE) was reported at a higher frequency in patients treated with ARIKAYCE plus background regimen (3.1%) compared to patients treated with a background regimen alone (0%). Most patients with hypersensitivity pneumonitis discontinued treatment with ARIKAYCE and received treatment with corticosteroids. If hypersensitivity pneumonitis occurs, discontinue ARIKAYCE and manage patients as medically appropriate.
Hemoptysis has been reported with the use of ARIKAYCE in the clinical trials. Hemoptysis was reported at a higher frequency in patients treated with ARIKAYCE plus background regimen (17.9%) compared to patients treated with a background regimen alone (12.5%). If hemoptysis occurs, manage patients as medically appropriate.
Bronchospasm has been reported with the use of ARIKAYCE in the clinical trials. Bronchospasm (reported as asthma, bronchial hyperreactivity, bronchospasm, dyspnea, dyspnea exertional, prolonged expiration, throat tightness, wheezing) was reported at a higher frequency in patients treated with ARIKAYCE plus background regimen (28.7%) compared to patients treated with a background regimen alone (10.7%). If bronchospasm occurs during the use of ARIKAYCE, treat patients as medically appropriate.
Exacerbations of underlying pulmonary disease has been reported with the use of ARIKAYCE in the clinical trials. Exacerbations of underlying pulmonary disease (reported as chronic obstructive pulmonary disease (COPD), infective exacerbation of COPD, infective exacerbation of bronchiectasis) have been reported at a higher frequency in patients treated with ARIKAYCE plus background regimen (14.8%) compared to patients treated with background regimen alone (9.8%). If exacerbations of underlying pulmonary disease occur during the use of ARIKAYCE, treat patients as medically appropriate.
Anaphylaxis and Hypersensitivity Reactions: Serious and potentially life-threatening hypersensitivity reactions, including anaphylaxis, have been reported in patients taking ARIKAYCE. Signs and symptoms include acute onset of skin and mucosal tissue hypersensitivity reactions (hives, itching, flushing, swollen lips/tongue/uvula), respiratory difficulty (shortness of breath, wheezing, stridor, cough), gastrointestinal symptoms (nausea, vomiting, diarrhea, crampy abdominal pain), and cardiovascular signs and symptoms of anaphylaxis (tachycardia, low blood pressure, syncope, incontinence, dizziness). Before therapy with ARIKAYCE is instituted, evaluate for previous hypersensitivity reactions to aminoglycosides. If anaphylaxis or a hypersensitivity reaction occurs, discontinue ARIKAYCE and institute appropriate supportive measures.
Ototoxicity has been reported with the use of ARIKAYCE in the clinical trials. Ototoxicity (including deafness, dizziness, presyncope, tinnitus, and vertigo) were reported with a higher frequency in patients treated with ARIKAYCE plus background regimen (17%) compared to patients treated with background regimen alone (9.8%). This was primarily driven by tinnitus (7.6% in ARIKAYCE plus background regimen vs 0.9% in the background regimen alone arm) and dizziness (6.3% in ARIKAYCE plus background regimen vs 2.7% in the background regimen alone arm). Closely monitor patients with known or suspected auditory or vestibular dysfunction during treatment with ARIKAYCE. If ototoxicity occurs, manage patients as medically appropriate, including potentially discontinuing ARIKAYCE.
Nephrotoxicity was observed during the clinical trials of ARIKAYCE in patients with MAC lung disease but not at a higher frequency than background regimen alone. Nephrotoxicity has been associated with the aminoglycosides. Close monitoring of patients with known or suspected renal dysfunction may be needed when prescribing ARIKAYCE.
Neuromuscular Blockade: Patients with neuromuscular disorders were not enrolled in ARIKAYCE clinical trials. Patients with known or suspected neuromuscular disorders, such as myasthenia gravis, should be closely monitored since aminoglycosides may aggravate muscle weakness by blocking the release of acetylcholine at neuromuscular junctions.
Embryo-Fetal Toxicity: Aminoglycosides can cause fetal harm when administered to a pregnant woman. Aminoglycosides, including ARIKAYCE, may be associated with total, irreversible, bilateral congenital deafness in pediatric patients exposed in utero. Patients who use ARIKAYCE during pregnancy, or become pregnant while taking ARIKAYCE should be apprised of the potential hazard to the fetus.
Contraindications: ARIKAYCE is contraindicated in patients with known hypersensitivity to any aminoglycoside.
Most Common Adverse Reactions: The most common adverse reactions in Trial 1 at an incidence ≥5% for patients using ARIKAYCE plus background regimen compared to patients treated with background regimen alone were dysphonia (47% vs 1%), cough (39% vs 17%), bronchospasm (29% vs 11%), hemoptysis (18% vs 13%), ototoxicity (17% vs 10%), upper airway irritation (17% vs 2%), musculoskeletal pain (17% vs 8%), fatigue and asthenia (16% vs 10%), exacerbation of underlying pulmonary disease (15% vs 10%), diarrhea (13% vs 5%), nausea (12% vs 4%), pneumonia (10% vs 8%), headache (10% vs 5%), pyrexia (7% vs 5%), vomiting (7% vs 4%), rash (6% vs 2%), decreased weight (6% vs 1%), change in sputum (5% vs 1%), and chest discomfort (5% vs 3%).
Drug Interactions: Avoid concomitant use of ARIKAYCE with medications associated with neurotoxicity, nephrotoxicity, and ototoxicity. Some diuretics can enhance aminoglycoside toxicity by altering aminoglycoside concentrations in serum and tissue. Avoid concomitant use of ARIKAYCE with ethacrynic acid, furosemide, urea, or intravenous mannitol.
Overdosage: Adverse reactions specifically associated with overdose of ARIKAYCE have not been identified. Acute toxicity should be treated with immediate withdrawal of ARIKAYCE, and baseline tests of renal function should be undertaken. Hemodialysis may be helpful in removing amikacin from the body. In all cases of suspected overdosage, physicians should contact the Regional Poison Control Center for information about effective treatment.
Patient example
Hear Dr Julie Philley briefly review a real-world example of a patient who had ARIKAYCE added to her multidrug regimen.
Recently in clinic, I saw a 67-year-old white female who was tall, slender, had nodular bronchiectasis, and multiple sputums which were positive for MAC. I placed her on a standard oral multidrug regimen, and she tolerated the regimen quite well. We checked sputums monthly, and despite the use of a correct regimen, she still remained culture positive for MAC at 6 months. I placed her on daily ARIKAYCE, according to the package insert, and several months later, her sputum converted to negative, and, she continues to do well on this multidrug regimen. She was very pleased with her progress and, of course, so was I.
IMPORTANT SAFETY INFORMATION
WARNING: RISK OF INCREASED RESPIRATORY ADVERSE REACTIONS
ARIKAYCE has been associated with an increased risk of respiratory adverse reactions, including hypersensitivity pneumonitis, hemoptysis, bronchospasm, and exacerbation of underlying pulmonary disease that have led to hospitalizations in some cases.
Hypersensitivity Pneumonitis has been reported with the use of ARIKAYCE in the clinical trials. Hypersensitivity pneumonitis (reported as allergic alveolitis, pneumonitis, interstitial lung disease, allergic reaction to ARIKAYCE) was reported at a higher frequency in patients treated with ARIKAYCE plus background regimen (3.1%) compared to patients treated with a background regimen alone (0%). Most patients with hypersensitivity pneumonitis discontinued treatment with ARIKAYCE and received treatment with corticosteroids. If hypersensitivity pneumonitis occurs, discontinue ARIKAYCE and manage patients as medically appropriate.
Hemoptysis has been reported with the use of ARIKAYCE in the clinical trials. Hemoptysis was reported at a higher frequency in patients treated with ARIKAYCE plus background regimen (17.9%) compared to patients treated with a background regimen alone (12.5%). If hemoptysis occurs, manage patients as medically appropriate.
Bronchospasm has been reported with the use of ARIKAYCE in the clinical trials. Bronchospasm (reported as asthma, bronchial hyperreactivity, bronchospasm, dyspnea, dyspnea exertional, prolonged expiration, throat tightness, wheezing) was reported at a higher frequency in patients treated with ARIKAYCE plus background regimen (28.7%) compared to patients treated with a background regimen alone (10.7%). If bronchospasm occurs during the use of ARIKAYCE, treat patients as medically appropriate.
Exacerbations of underlying pulmonary disease has been reported with the use of ARIKAYCE in the clinical trials. Exacerbations of underlying pulmonary disease (reported as chronic obstructive pulmonary disease (COPD), infective exacerbation of COPD, infective exacerbation of bronchiectasis) have been reported at a higher frequency in patients treated with ARIKAYCE plus background regimen (14.8%) compared to patients treated with background regimen alone (9.8%). If exacerbations of underlying pulmonary disease occur during the use of ARIKAYCE, treat patients as medically appropriate.
Anaphylaxis and Hypersensitivity Reactions: Serious and potentially life-threatening hypersensitivity reactions, including anaphylaxis, have been reported in patients taking ARIKAYCE. Signs and symptoms include acute onset of skin and mucosal tissue hypersensitivity reactions (hives, itching, flushing, swollen lips/tongue/uvula), respiratory difficulty (shortness of breath, wheezing, stridor, cough), gastrointestinal symptoms (nausea, vomiting, diarrhea, crampy abdominal pain), and cardiovascular signs and symptoms of anaphylaxis (tachycardia, low blood pressure, syncope, incontinence, dizziness). Before therapy with ARIKAYCE is instituted, evaluate for previous hypersensitivity reactions to aminoglycosides. If anaphylaxis or a hypersensitivity reaction occurs, discontinue ARIKAYCE and institute appropriate supportive measures.
Ototoxicity has been reported with the use of ARIKAYCE in the clinical trials. Ototoxicity (including deafness, dizziness, presyncope, tinnitus, and vertigo) were reported with a higher frequency in patients treated with ARIKAYCE plus background regimen (17%) compared to patients treated with background regimen alone (9.8%). This was primarily driven by tinnitus (7.6% in ARIKAYCE plus background regimen vs 0.9% in the background regimen alone arm) and dizziness (6.3% in ARIKAYCE plus background regimen vs 2.7% in the background regimen alone arm). Closely monitor patients with known or suspected auditory or vestibular dysfunction during treatment with ARIKAYCE. If ototoxicity occurs, manage patients as medically appropriate, including potentially discontinuing ARIKAYCE.
Nephrotoxicity was observed during the clinical trials of ARIKAYCE in patients with MAC lung disease but not at a higher frequency than background regimen alone. Nephrotoxicity has been associated with the aminoglycosides. Close monitoring of patients with known or suspected renal dysfunction may be needed when prescribing ARIKAYCE.
Neuromuscular Blockade: Patients with neuromuscular disorders were not enrolled in ARIKAYCE clinical trials. Patients with known or suspected neuromuscular disorders, such as myasthenia gravis, should be closely monitored since aminoglycosides may aggravate muscle weakness by blocking the release of acetylcholine at neuromuscular junctions.
Embryo-Fetal Toxicity: Aminoglycosides can cause fetal harm when administered to a pregnant woman. Aminoglycosides, including ARIKAYCE, may be associated with total, irreversible, bilateral congenital deafness in pediatric patients exposed in utero. Patients who use ARIKAYCE during pregnancy, or become pregnant while taking ARIKAYCE should be apprised of the potential hazard to the fetus.
Contraindications: ARIKAYCE is contraindicated in patients with known hypersensitivity to any aminoglycoside.
Most Common Adverse Reactions: The most common adverse reactions in Trial 1 at an incidence ≥5% for patients using ARIKAYCE plus background regimen compared to patients treated with background regimen alone were dysphonia (47% vs 1%), cough (39% vs 17%), bronchospasm (29% vs 11%), hemoptysis (18% vs 13%), ototoxicity (17% vs 10%), upper airway irritation (17% vs 2%), musculoskeletal pain (17% vs 8%), fatigue and asthenia (16% vs 10%), exacerbation of underlying pulmonary disease (15% vs 10%), diarrhea (13% vs 5%), nausea (12% vs 4%), pneumonia (10% vs 8%), headache (10% vs 5%), pyrexia (7% vs 5%), vomiting (7% vs 4%), rash (6% vs 2%), decreased weight (6% vs 1%), change in sputum (5% vs 1%), and chest discomfort (5% vs 3%).
Drug Interactions: Avoid concomitant use of ARIKAYCE with medications associated with neurotoxicity, nephrotoxicity, and ototoxicity. Some diuretics can enhance aminoglycoside toxicity by altering aminoglycoside concentrations in serum and tissue. Avoid concomitant use of ARIKAYCE with ethacrynic acid, furosemide, urea, or intravenous mannitol.
Overdosage: Adverse reactions specifically associated with overdose of ARIKAYCE have not been identified. Acute toxicity should be treated with immediate withdrawal of ARIKAYCE, and baseline tests of renal function should be undertaken. Hemodialysis may be helpful in removing amikacin from the body. In all cases of suspected overdosage, physicians should contact the Regional Poison Control Center for information about effective treatment.
INDICATION
LIMITED POPULATION: ARIKAYCE® is indicated in adults, who have limited or no alternative treatment options, for the treatment of Mycobacterium avium complex (MAC) lung disease as part of a combination antibacterial drug regimen in patients who do not achieve negative sputum cultures after a minimum of 6 consecutive months of a multidrug background regimen therapy. As only limited clinical safety and effectiveness data for ARIKAYCE are currently available, reserve ARIKAYCE for use in adults who have limited or no alternative treatment options. This drug is indicated for use in a limited and specific population of patients.
This indication is approved under accelerated approval based on achieving sputum culture conversion (defined as 3 consecutive negative monthly sputum cultures) by Month 6. Clinical benefit has not yet been established. Continued approval for this indication may be contingent upon verification and description of clinical benefit in confirmatory trials.
Limitation of Use: ARIKAYCE has only been studied in patients with refractory MAC lung disease defined as patients who did not achieve negative sputum cultures after a minimum of 6 consecutive months of a multidrug background regimen therapy. The use of ARIKAYCE is not recommended for patients with non-refractory MAC lung disease.
Experts discuss: 2020 NTM guidelines recommendations and treatment options for MAC lung disease
Drs David Griffith, Charles Daley, Julie Philley, and Shannon Kasperbauer do a deep dive of the NTM Guidelines in this expert roundtable.
Welcome to an expert discussion of the new 2020 NTM guideline recommendations and treatment options for MAC lung disease.
Hello. My name is David Griffith from National Jewish Health. And I am a member of the 2020 NTM Guidelines Committee. For today's discussion, I'm joined by several NTM expert colleagues-- Dr. Charles Daley, chief of the division of mycobacterial and respiratory infections at National Jewish Health in Denver, Dr. Julie Philley from the University of Texas Health Science Center at Tyler, Texas, and Dr. Shannon Kasperbauer also from National Jewish Health. I want to thank you all for being here.
Let's discuss the 2020 NTM guideline recommendation for ARIKAYCE or amikacin liposome inhalation suspension. Adding ARIKAYCE is strongly recommended in patients who fail six months of initial treatment as defined by persistently positive sputum cultures for MAC at six months.
As noted, it is a strong recommendation, which was rigorously derived with moderate certainty and estimates of effect. The recommendation is add ARIKAYCE to treatment regimens in patients who remain culture-positive at six months. ARIKAYCE is not approved for use as part of initial treatment regimens in newly diagnosed patients. So why did ARIKAYCE receive a strong recommendation? Julie, let's start with you.
Well, thanks, Dave. I think it's really important to note that the strong recommendation came from two robust randomized controlled trials that were conducted with rigorous investigation methods. I think that it was really an important trial with a lot of data that I think is supportive of using ARIKAYCE in refractory disease. Not only was it robust, I think that we, as health experts and NTM experts, believe the results. And the results are really important for this treatment-refractory population.
Shannon, would you like to add anything?
No, I agree. It's really the quality of the evidence. And this is a disease state where we have a paucity of randomized controlled trials. And the fact that we have two that both had similar outcomes that reliably validated one another, this is important information for our patients with treatment-refractory MAC.
Chuck, any thoughts?
Well, we're only four strong recommendations out of the 31 that were made in the guidelines and two related to MAC, and, of course, this was one of them. So clearly, we work in a field for which we don't have as much evidence as we would like. So all the more that this was fairly unique to receive a strong recommendation.
Yeah. ARIKAYCE is the first and only FDA-approved product for refractory MAC lung disease. It is for a limited population. ARIKAYCE is indicated in adults who have limited or no alternative treatment options for the treatment of Mycobacterium Avium Complex, or MAC, lung disease as part of a combination antibacterial drug regimen in patients who do not achieve negative sputum cultures after a minimum of six consecutive months of multidrug background regimen therapy.
As only limited clinical safety and effectiveness data for ARIKAYCE are currently available, reserve ARIKAYCE for use in adults who have limited or no alternative treatment options. This drug is indicated for use in a limited and specific population of patients. This indication is approved under accelerated approval based on achieving sputum culture conversion defined as three consecutive negative monthly sputum cultures by month six.
Clinical benefit has not yet been established. Continued approval for this indication may be contingent upon verification and description of clinical benefit in confirmatory trials. ARIKAYCE has only been studied in patients with refractory MAC lung disease defined as patients who did not achieve negative sputum cultures after a minimum of six consecutive months of a multidrug background regimen therapy. The use of ARIKAYCE is not recommended for patients with nonrefractory MAC lung disease ARIKAYCE is contraindicated in patients with a known hypersensitivity to any aminoglycoside.
There is also a boxed warning that accompanies ARIKAYCE. Risk of increased respiratory adverse reactions. ARIKAYCE has been associated with an increased risk of respiratory adverse reactions, including hypersensitivity pneumonitis, hemoptysis, bronchospasm, and exacerbation of underlying pulmonary disease that have led to hospitalizations in some cases.
This is the study design for the pivotal phase III CONVERT trial, which, as you can see, is labeled as a rigorous study design, which I think is an accurate description. The population in this trial were adults with treatment-refractory MAC lung disease.
At screening, they were randomized in a 2-to-1 fashion to receive either ARIKAYCE daily plus the background regimen. That's the two. Or the one that continue on the background regimen alone, you can see there were 224 patients in the ARIKAYCE arm and 112 in the background regimen.
The study design required patients to take ARIKAYCE and background regimen or background regimen alone for 12 months from the first month of conversion. The primary endpoint was the percentage of patients with culture conversion by month six. There were two select secondary endpoints-- the six-minute walk and the St George's Respiratory Questionnaire.
In the off-treatment phase, patients were analyzed at three months after completing therapy. And, again, at month 28 at the end of study visit, in both arms, there was a 12 months off-treatment follow-up.
Just a quick note. When patients converted their sputum and continued on therapy, the maintenance of culture negativity was assessed as sustainability of culture conversion. Once treatment was stopped, the maintenance of culture negativity was described as durability of culture conversion.
Before going into this culture conversion slide, I think it's worth emphasizing a couple of points. First, as noted, patients had to have failed at least six months of guideline-based therapy with persistently positive sputum cultures at six months.
But it is important to remember that most of these patients had been diagnosed with MAC several years prior to participation in this study and had been treated for MAC at least once and perhaps multiple times before entering the study. These were not patients who had just come along, gotten six months of treatment, failed that treatment, and then put on the study. These were patients who were truly refractory to MAC therapy.
The second important point, which has to do with the rigorous execution of this study, was that at each point where sputum was collected, three specimens were collected. So to meet the definition of sputum culture conversion, patients had to have negative sputum cultures at three consecutive visits.
So with three cultures collected at each visit, that means those patients had to have nine negative sputum cultures to be considered culture-negative or a culture converter. That is quite a high bar for determining culture negativity.
The other thing to remember is that in order for a patient to be considered a culture converter by six months, they had to have their first negative sputum culture by month four. And that's why on this graph, you see that the data ends at month four. If a patient had their first negative sputum culture at month five, they could not have met the definition of sputum culture conversion as defined in the study protocol.
So what this graph shows is that by month four, 29% of the patients who were receiving ARIKAYCE plus their background regimen converted their sputum compared to 9% of the patients who had continued their background regimen alone. You can see that was a three-fold increase, which was highly significant statistically. So why is 29% increase in culture conversion significant in patients who you are refractory? Shannon, any thoughts?
Well, I think you have to first put it into context of who the sponsor was studying, and these were treatment-refractory patients. But if you look at the details of the study, the average duration of MAC lung disease in these patients was 4.5 years.
Yes. Julie, could you describe the typical patient enrolled at your center in this study?
Sure, Dave. These were patients that were on therapy for years with little hope of culture conversion. So you can imagine our surprise and certainly surprise all over the United States that when we saw this data, it felt like, wow, this is very impactful. These were not patients that I would have anticipated would have had much of a shot at converting to negative. And yet nearly a third of them did.
Yeah, it's important to emphasize that. That 30% would be patients who would still be on treatment had it not been for the intervention.
Absolutely.
Now let's go to Dr. Philley who will discuss the select secondary endpoints and safety data from the CONVERT trial. Julie?
Thanks, Dave. You alluded to this select secondary endpoints in this study in your previous slide. The secondary endpoints of the change from baseline in the six-minute walk test and the St. George Respiratory Questionnaire were evaluated but did not demonstrate clinical benefit by month six in the study.
Secondary endpoints in the decision of which secondary endpoints to measure can be difficult in these types of studies. But it is important to note that these did not reach statistical clinical significance. This is a list of adverse reactions occurring in more than 5% of patients. The most common adverse reaction was dysphonia or changes in the voice. This occurred in 47% of the ARIKAYCE and background regimen arm versus 1% in the background regimen alone.
The other adverse reactions that were common in the study are listed here and include cough, which was also very common, bronchospasm, hemoptysis, ototoxicity, upper airway irritation, musculoskeletal pain, fatigue, and flare-ups of underlying pulmonary diseases.
Other reactions which were also noted in the study included diarrhea, nausea, pneumonia, headache, low-grade fevers, vomiting, rash, weight loss or changes in the weight, changes in sputum, and chest discomfort.
There is a risk of increased respiratory adverse reactions. ARIKAYCE has been associated with an increased risk of respiratory adverse reactions, including hypersensitivity pneumonitis, hemoptysis, bronchospasm, and exacerbation of underlying pulmonary disease, that have led to hospitalization in some cases.
Hypersensitivity pneumonitis has been reported with the use of ARIKAYCE in the clinical trials. Hypersensitivity pneumonitis reported as allergic alveolitis, pneumonitis, interstitial lung disease. Allergic reaction to ARIKAYCE was reported at a higher frequency in patients treated with ARIKAYCE plus background regimen compared to patients treated with a background regimen alone.
Most patients with hypersensitivity pneumonitis discontinued treatment with ARIKAYCE and received treatment with corticosteroids. If hypersensitivity pneumonitis occurs, discontinue ARIKAYCE and manage patients as medically appropriate.
Hemoptysis has been reported with the use of ARIKAYCE in the clinical trials. Hemoptysis was reported at a higher frequency in patients treated with ARIKAYCE plus background regimen compared in patients treated with a background regimen alone. If hemoptysis occurs, manage patients as medically appropriate.
Bronchospasm has been reported with the use of ARIKAYCE in the clinical trials. Bronchospasm reported as asthma, bronchial hyperreactivity, bronchospasm, dyspnea, exertional dyspnea, prolonged exploration, throat tightness, wheezing was reported at a higher frequency in patients treated with ARIKAYCE plus background regimen compared to patients treated with a background regimen alone. If bronchospasm occurs during the use of ARIKAYCE, treat patients as medically appropriate.
Exacerbations of underlying pulmonary disease has been reported with the use of ARIKAYCE in the clinical trials. Exacerbations of underlying pulmonary disease reported as chronic obstructive pulmonary disease, infective exacerbations of COPD, infective exacerbation of bronchiectasis have been reported at a higher frequency of patients treated with ARIKAYCE plus background regimen compared to patients treated with a background regimen alone. If exacerbations of underlying pulmonary disease occur during the use of ARIKAYCE, treat patients as medically appropriate.
Anaphylaxis and hypersensitivity reactions. Serious and potentially life-threatening hypersensitivity reactions, including anaphylaxis, have been reported in patients taking ARIKAYCE. Signs and symptoms include acute onset of skin and mucosal tissue, hypersensitivity reactions, hives, itching, flushing, swollen lips, tongue, respiratory difficulty, shortness of breath, wheezing, strider, cough.
Gastrointestinal symptoms-- nausea, vomiting, diarrhea, crampy abdominal pain. And cardiovascular signs and symptoms of anaphylaxis-- tachycardia, low blood pressure, syncope, incontinence, dizziness. Before therapy with ARIKAYCE is instituted, evaluate for previous hypersensitivity reactions to aminoglycosides. If anaphylaxis or a hypersensitivity reaction occurs, discontinue ARIKAYCE and institute appropriate supportive measures.
Ototoxicity has been reported with the use of ARIKAYCE in clinical trials. Ototoxicity, including deafness, dizziness, pre-syncope, tinnitus, and vertigo were reported with a higher frequency in patients treated with ARIKAYCE plus background regimen compared to patients treated with background regimen alone.
This was primarily driven by tinnitus and dizziness. Closely monitor patients with known or suspected auditory or vestibular dysfunction during treatment with ARIKAYCE. If ototoxicity occurs, manage patients as medically appropriate, including potentially discontinuing ARIKAYCE.
Nephrotoxicity was observed during the clinical trials of ARIKAYCE in patients with MAC lung disease but not at a higher frequency than background regimen alone. Nephrotoxicity has been associated with aminoglycosides. Close monitoring of patients with known or suspected renal dysfunction may be needed when prescribing ARIKAYCE.
Neuromuscular blockade. Patients with neuromuscular disorders were not enrolled in ARIKAYCE clinical trials. Patients with known or suspected neuromuscular disorders, such as myasthenia gravis, should be closely monitored since aminoglycosides may aggravate muscular weakness by blocking the release of acetylcholine at neuromuscular junctions.
Aminoglycosides can cause fetal harm when administered to a pregnant woman. Aminoglycosides, including ARIKAYCE, may be associated with total irreversible bilateral congenital deafness in pediatric patients exposed in utero. Patients who use ARIKAYCE during pregnancy or become pregnant while taking ARIKAYCE should be apprised of the potential harm to the fetus.
ARIKAYCE is contraindicated in patients with known hypersensitivity to any aminoglycoside. The most common adverse reactions in a trial at the incidence of greater than or equal to 5% for patients using ARIKAYCE plus background regimen compared to patients treated with background regimen alone were dysphonia, cough, bronchospasm, hemoptysis, ototoxicity, upper airway irritation, musculoskeletal pain, fatigue and asthenia, exacerbation of underlying pulmonary disease, diarrhea, nausea, pneumonia, headache, pyrexia, vomiting, rash, decreased weight, change in sputum, and chest discomfort.
Avoid concomitant use of ARIKAYCE with medications associated with neurotoxicity, nephrotoxicity, and ototoxicity. Some diuretics can enhance aminoglycoside toxicity by altering aminoglycoside concentrations in serum and tissue. Avoid concomitant use of ARIKAYCE with ethacrynic acid, furosemide, urea, or intravenous mannitol.
Adverse reactions specifically associated with overdose of ARIKAYCE have not been identified. Acute toxicity should be treated with immediate withdrawal of ARIKAYCE, and baseline test of renal function should be undertaken. Hemodialysis may be helpful in removing amikacin from the body. In all cases of suspected overdosage, physicians should contact the regional poison control center for information about effective treatment.
So I'd like to touch on the role of ARIKAYCE in the management of MAC lung disease. Where it becomes important is that at month six, if a patient remains culture-positive, it is recommended to add ARIKAYCE. Again, if your sputum remain culture-positive at six months despite a treatment with multidrug regimen for MAC lung disease, the addition of ARIKAYCE is recommended. You should continue MAC treatment for 12 months after cultures have converted to negative.
So thinking about ARIKAYCE, how we monitor patients, how we use the drug, Shannon, how often do you monitor patients on ARIKAYCE?
Julie, from my experience, the frequency of visits really stays the same as I would be monitoring a patient on their usual background regimen. So I typically see patients every two to three months in clinic. The laboratory monitoring does not change.
You mentioned monitoring patients, thinking about potential side effects, adverse reactions. Dave, how do you set expectations with your patients when you talk about these things like, for example, when you talk about potential side effects?
Yeah, it's an extremely important topic, particularly when you have an adverse event such as dysphonia, which occurs in about half the patients who take the drug. So it is important to go through the common side effects with the patient but equally important to discuss with them how they're managed.
And so, for instance, with dysphonia, to talk about steps that can be taken if it occurs. It is absolutely important to let patients understand that there can be problems when they take this drug.
When we treat refractory patients, they're often on a multitude of drugs for a long period of time. Chuck, what do you think the impact is of having a treatment option recommended for patients with refractory disease? And why do you think it's important?
Well, this is a group of patients that have usually been treated for a long time, may have suffered complications. And when they're not converting, we're talking to them about next steps. And we really haven't had many next steps before.
So now that we have an actual intervention that has been rigorously proven to be effective, we can now sit before our patients and say, here's what we're going to do, and provide them with very hard evidence for what they can expect in terms of culture conversion. And that is really remarkable for us in this field.
I couldn't agree more. And thank you so much, Chuck. And thanks to each of our panelists. We appreciate you for being here and joining us. And thank you for those of you out there. We hope you found this discussion helpful. Please visit ARIKAYCEhcp.com for more information.
ARIKAYCE clinical data: CONVERT
Drs David Griffith and Nicole Lapinel explore the CONVERT data and discuss its importance to patients with MAC lung disease
ARIKAYCE® (amikacin liposome inhalation suspension) is the first and only FDA-approved treatment for refractory Mycobacterium avium complex (MAC) lung disease in adults who remain culture-positive after a minimum of 6 consecutive months of a multidrug background regimen therapy. ARIKAYCE should be used in combination with an antibacterial drug regimen in adults who have limited or no alternative treatment options.1
Hi, my name is Dr David Griffith, and I am a pulmonologist and professor of medicine at National Jewish Health. In Chapter 1 of this video, my colleague, Dr Nicole Lapinel, and I will review the robust clinical data from the CONVERT study, including responder data, and will discuss the importance of adding ARIKAYCE in patients with refractory MAC lung disease.
Hello, my name is Dr Nicole Lapinel, and I am a pulmonologist and assistant professor of medicine with Northwell Health in New York. After discussing the results of the CONVERT data in Chapter 1, Chapter 2 will highlight the results from the open-label safety extension study of CONVERT, which is referred to as the INS-312 study.
The CONVERT study was an open-label, multicenter trial that enrolled 336 patients with refractory MAC lung disease. Patients were randomized 2:1 to receive either ARIKAYCE plus standard therapy or standard therapy alone.1
The study protocol defined refractory MAC lung disease as persistently positive cultures after at least 6 months of standard guideline-based therapy.1 This is consistent with the latest 2020 multisociety NTM Treatment Guidelines definition.2
The primary endpoint was culture conversion by Month 6. This was defined as 3 consecutive negative sputum cultures, the first of which had to be achieved by Month 4.1
The key secondary endpoints were1
- Change from baseline in 6-minute walk test and the St George’s Respiratory Questionnaire at Month 6
- Culture conversion 12 months after initial conversion and 3 months off treatment The exploratory endpoint was culture conversion 12 months off treatment.3
For the primary endpoint, although culture conversion rates were similar between both arms at baseline, the arms started to have a clear separation at Month 1. This separation continued to increase through Months 3 and 4 in the ARIKAYCE plus standard therapy arm and plateaued for the standardtherapy- alone arm.1
29% of patients receiving ARIKAYCE plus standard therapy achieved culture conversion by Month 6 compared with 8.9% of patients on standard therapy alone, a 3-fold increase. This was found to be statistically significant. The secondary endpoints of change from baseline in 6-minute walk test and St George’s Respiratory Questionnaire did not demonstrate clinical benefit by Month 6.1
When evaluating culture conversion in the intent-to-treat, or ITT, population, you can see that 18.3% of patients receiving ARIKAYCE plus standard therapy maintained culture conversion by Month 12 compared with 2.7% of patients receiving standard therapy alone.1
When taken off treatment for 3 months, only patients receiving ARIKAYCE plus standard therapy continued to maintain culture conversion.1
Both culture conversion endpoints were statistically significant in favor of ARIKAYCE plus standard therapy.1
The most common adverse events in the patients who received ARIKAYCE plus standard therapy were dysphonia, cough, bronchospasm, hemoptysis, musculoskeletal pain, upper airway irritation, ototoxicity, fatigue and asthenia, exacerbation of underlying pulmonary disease, diarrhea, nausea, and headache.1
Dr Griffith, given your extensive experience in this field, can you please explain why these data are compelling?
Well, first we have only had observational data for the past few decades, and now we have evidence from 2 randomized trials.
- These patients in the CONVERT trial went through years where they were treated without conversion
- Culture conversion, as defined by the study protocol, was rigorous4
- 29% of patients receiving ARIKAYCE plus standard therapy achieved culture conversion by Month 6 compared with 8.9% of patients on standard therapy alone1
- With ARIKAYCE, you see many patients achieve culture conversion for 12 months on therapy, and some continue to achieve culture conversion for 3 months off therapy1
- For patients who do achieve culture conversion, maintaining it is the goal
Let’s take a deeper look at the patients who achieved the primary endpoint of culture conversion and who initially responded to treatment.
In this population more than half of the patients receiving ARIKAYCE plus standard therapy maintained culture conversion 12 months after initial culture conversion compared with about one-third of patients on standard therapy alone.3
Similarly, for the culture conversion rate 3 months off treatment, more than half of the patients continued to have negative sputum cultures compared with no patients in the standard-therapy-alone arm.3
In addition, nearly half of the responder population maintained culture conversion 12 months off treatment compared with no patients receiving standard therapy alone.3
Dr Lapinel, why is maintaining culture conversion so important in these patients?
Maintaining culture conversion is important for a few reasons.
- These patients have usually been on standard antibiotic therapy for years without being able to achieve conversion
- ARIKAYCE provides patients with refractory MAC lung disease the opportunity to achieve and maintain culture conversion. As you mentioned, this is often difficult for these patients
- This should help reinforce the potential that ARIKAYCE can have in patients with refractory MAC lung disease
- Furthermore, as an antibacterial infection, we consider improvement to be measured by an objective assessment. For patients with refractory MAC lung disease, that translates to achieving and maintaining culture conversion
The most common adverse reactions occurring in ≥5% of patients1 were respiratory in nature and more frequent in patients receiving ARIKAYCE plus standard therapy compared with patients receiving standard therapy alone.4
Adverse reactions in order of most to least frequent included dysphonia, cough, bronchospasm, hemoptysis, musculoskeletal pain, upper airway irritation, ototoxicity, fatigue and asthenia, exacerbation of underlying pulmonary disease diarrhea, nausea, headache, pneumonia, pyrexia, weight decreased, vomiting, rash, change in sputum, and chest discomfort.1
Furthermore, the emergence of most treatment-emergent adverse events, or TEAEs, was reported within the first month of the ARIKAYCE plus standard therapy arm. The incidence of new-onset TEAEs declined thereafter, similar to the standardtherapy- alone arm.4
Discontinuations due to TEAEs primarily occurred early in the trial and declined over time.5
The first month of ARIKAYCE is especially important for monitoring TEAEs to be able to address these AEs early.
In summary, in patients with refractory MAC lung disease, the addition of ARIKAYCE to standard therapy helped achieve culture conversion with a 3-fold difference compared with patients on standard therapy alone.1 Furthermore, ARIKAYCE maintained culture conversion in many of these patients1 and is the 2020 NTM Guidelines–recommended option to add to standard therapy for patients with refractory MAC lung disease.2
These data help reinforce adding ARIKAYCE in our patients with refractory MAC lung disease at 6 months,2 since it can help patients achieve and maintain culture conversion.1
In Chapter 2, we will discuss the safety results of ARIKAYCE from the open-label extension study.6
INS-312 was an open-label safety study that enrolled patients who did not achieve culture conversion by Month 6 in the CONVERT study or who had recurrent MAC lung infection confirmed by Month 8.6
In this study, patients who received ARIKAYCE plus standard therapy in the CONVERT study were assigned to the prior-ARIKAYCE arm and patients who were on standard therapy alone were placed in the ARIKAYCEnaive arm. Both arms, regardless of prior ARIKAYCE exposure, received ARIKAYCE plus standard therapy.6
The primary endpoint was the frequency of TEAEs, including those leading to withdrawal, those of special interest, and serious TEAEs, assessed at Month 12.6
The secondary endpoints were culture conversion and change from baseline in the 6-minute walk test at Month 6 and Month 12.6
No new safety signals were detected with up to 20 months of exposure to ARIKAYCE, and the adverse reactions were consistent with the CONVERT study for both arms.6
The most common adverse reactions were respiratory in nature and included dysphonia, cough, pulmonary exacerbation, dyspnea, fatigue, hemoptysis, infective exacerbation of bronchiectasis, nausea, diarrhea, and nasopharyngitis.6
Dr Griffith, what do these data mean for health care professionals treating patients on ARIKAYCE?
It is encouraging for HCPs to know that there are data for patients who continue treatment with ARIKAYCE.
What was the duration of therapy for patients in the trial?
Patients in the trial were on therapy for 12 months from the date of their first culture conversion.
The secondary efficacy endpoint demonstrated that patients in the prior-ARIKAYCE arm achieved culture conversion with continued extended use of ARIKAYCE beyond 6 months. 13.7% of patients achieved culture conversion by Month 12, which was equivalent to approximately 16 to 18 months of ARIKAYCE exposure.6
In the ARIKAYCE-naive arm, 26.7% of patients achieved culture conversion by Month 6.6
In summary, the INS-312 study demonstrated that no new safety signals were detected and that some patients who did not convert their cultures by Month 6 in the CONVERT study achieved culture conversion with extended use of ARIKAYCE.6
These data from the open-label safety study demonstrated that ARIKAYCE has a consistent safety profile with the CONVERT study and patients had an increased chance of achieving culture conversion with extended treatment.6
Monitoring ARIKAYCE treatment
Dr Wendi Drummond shares how she approaches the frequency of sputum culture collection, surveillance computed tomography scans, patient follow-up, and more.
When I add ARIKAYCE to their regimen, we discuss that we will be obtaining monthly sputums to assess their response to treatment because we are looking for sputum conversion to negative, and then, just as part of their multidrug regimen, I do surveillance CTs at whatever interval I think is specific and needed for that patient. Once my patients start on ARIKAYCE therapy, I typically see them within a month after starting treatment to touch base and see how they’re tolerating the medication, and then I see them every 3 months thereafter at which time they have routine monitoring labs, as well as routine audiograms.
IMPORTANT SAFETY INFORMATION
WARNING: RISK OF INCREASED RESPIRATORY ADVERSE REACTIONS
ARIKAYCE has been associated with an increased risk of respiratory adverse reactions, including hypersensitivity pneumonitis, hemoptysis, bronchospasm, and exacerbation of underlying pulmonary disease that have led to hospitalizations in some cases.
Hypersensitivity Pneumonitis has been reported with the use of ARIKAYCE in the clinical trials. Hypersensitivity pneumonitis (reported as allergic alveolitis, pneumonitis, interstitial lung disease, allergic reaction to ARIKAYCE) was reported at a higher frequency in patients treated with ARIKAYCE plus background regimen (3.1%) compared to patients treated with a background regimen alone (0%). Most patients with hypersensitivity pneumonitis discontinued treatment with ARIKAYCE and received treatment with corticosteroids. If hypersensitivity pneumonitis occurs, discontinue ARIKAYCE and manage patients as medically appropriate.
Hemoptysis has been reported with the use of ARIKAYCE in the clinical trials. Hemoptysis was reported at a higher frequency in patients treated with ARIKAYCE plus background regimen (17.9%) compared to patients treated with a background regimen alone (12.5%). If hemoptysis occurs, manage patients as medically appropriate.
Bronchospasm has been reported with the use of ARIKAYCE in the clinical trials. Bronchospasm (reported as asthma, bronchial hyperreactivity, bronchospasm, dyspnea, dyspnea exertional, prolonged expiration, throat tightness, wheezing) was reported at a higher frequency in patients treated with ARIKAYCE plus background regimen (28.7%) compared to patients treated with a background regimen alone (10.7%). If bronchospasm occurs during the use of ARIKAYCE, treat patients as medically appropriate.
Exacerbations of underlying pulmonary disease has been reported with the use of ARIKAYCE in the clinical trials. Exacerbations of underlying pulmonary disease (reported as chronic obstructive pulmonary disease (COPD), infective exacerbation of COPD, infective exacerbation of bronchiectasis) have been reported at a higher frequency in patients treated with ARIKAYCE plus background regimen (14.8%) compared to patients treated with background regimen alone (9.8%). If exacerbations of underlying pulmonary disease occur during the use of ARIKAYCE, treat patients as medically appropriate.
Anaphylaxis and Hypersensitivity Reactions: Serious and potentially life-threatening hypersensitivity reactions, including anaphylaxis, have been reported in patients taking ARIKAYCE. Signs and symptoms include acute onset of skin and mucosal tissue hypersensitivity reactions (hives, itching, flushing, swollen lips/tongue/uvula), respiratory difficulty (shortness of breath, wheezing, stridor, cough), gastrointestinal symptoms (nausea, vomiting, diarrhea, crampy abdominal pain), and cardiovascular signs and symptoms of anaphylaxis (tachycardia, low blood pressure, syncope, incontinence, dizziness). Before therapy with ARIKAYCE is instituted, evaluate for previous hypersensitivity reactions to aminoglycosides. If anaphylaxis or a hypersensitivity reaction occurs, discontinue ARIKAYCE and institute appropriate supportive measures.
Ototoxicity has been reported with the use of ARIKAYCE in the clinical trials. Ototoxicity (including deafness, dizziness, presyncope, tinnitus, and vertigo) were reported with a higher frequency in patients treated with ARIKAYCE plus background regimen (17%) compared to patients treated with background regimen alone (9.8%). This was primarily driven by tinnitus (7.6% in ARIKAYCE plus background regimen vs 0.9% in the background regimen alone arm) and dizziness (6.3% in ARIKAYCE plus background regimen vs 2.7% in the background regimen alone arm). Closely monitor patients with known or suspected auditory or vestibular dysfunction during treatment with ARIKAYCE. If ototoxicity occurs, manage patients as medically appropriate, including potentially discontinuing ARIKAYCE.
Nephrotoxicity was observed during the clinical trials of ARIKAYCE in patients with MAC lung disease but not at a higher frequency than background regimen alone. Nephrotoxicity has been associated with the aminoglycosides. Close monitoring of patients with known or suspected renal dysfunction may be needed when prescribing ARIKAYCE.
Neuromuscular Blockade: Patients with neuromuscular disorders were not enrolled in ARIKAYCE clinical trials. Patients with known or suspected neuromuscular disorders, such as myasthenia gravis, should be closely monitored since aminoglycosides may aggravate muscle weakness by blocking the release of acetylcholine at neuromuscular junctions.
Embryo-Fetal Toxicity: Aminoglycosides can cause fetal harm when administered to a pregnant woman. Aminoglycosides, including ARIKAYCE, may be associated with total, irreversible, bilateral congenital deafness in pediatric patients exposed in utero. Patients who use ARIKAYCE during pregnancy, or become pregnant while taking ARIKAYCE should be apprised of the potential hazard to the fetus.
Contraindications: ARIKAYCE is contraindicated in patients with known hypersensitivity to any aminoglycoside.
Most Common Adverse Reactions: The most common adverse reactions in Trial 1 at an incidence ≥5% for patients using ARIKAYCE plus background regimen compared to patients treated with background regimen alone were dysphonia (47% vs 1%), cough (39% vs 17%), bronchospasm (29% vs 11%), hemoptysis (18% vs 13%), ototoxicity (17% vs 10%), upper airway irritation (17% vs 2%), musculoskeletal pain (17% vs 8%), fatigue and asthenia (16% vs 10%), exacerbation of underlying pulmonary disease (15% vs 10%), diarrhea (13% vs 5%), nausea (12% vs 4%), pneumonia (10% vs 8%), headache (10% vs 5%), pyrexia (7% vs 5%), vomiting (7% vs 4%), rash (6% vs 2%), decreased weight (6% vs 1%), change in sputum (5% vs 1%), and chest discomfort (5% vs 3%).
Drug Interactions: Avoid concomitant use of ARIKAYCE with medications associated with neurotoxicity, nephrotoxicity, and ototoxicity. Some diuretics can enhance aminoglycoside toxicity by altering aminoglycoside concentrations in serum and tissue. Avoid concomitant use of ARIKAYCE with ethacrynic acid, furosemide, urea, or intravenous mannitol.
Overdosage: Adverse reactions specifically associated with overdose of ARIKAYCE have not been identified. Acute toxicity should be treated with immediate withdrawal of ARIKAYCE, and baseline tests of renal function should be undertaken. Hemodialysis may be helpful in removing amikacin from the body. In all cases of suspected overdosage, physicians should contact the Regional Poison Control Center for information about effective treatment.
INDICATION
LIMITED POPULATION: ARIKAYCE® is indicated in adults, who have limited or no alternative treatment options, for the treatment of Mycobacterium avium complex (MAC) lung disease as part of a combination antibacterial drug regimen in patients who do not achieve negative sputum cultures after a minimum of 6 consecutive months of a multidrug background regimen therapy. As only limited clinical safety and effectiveness data for ARIKAYCE are currently available, reserve ARIKAYCE for use in adults who have limited or no alternative treatment options. This drug is indicated for use in a limited and specific population of patients.
This indication is approved under accelerated approval based on achieving sputum culture conversion (defined as 3 consecutive negative monthly sputum cultures) by Month 6. Clinical benefit has not yet been established. Continued approval for this indication may be contingent upon verification and description of clinical benefit in confirmatory trials.
Limitation of Use: ARIKAYCE has only been studied in patients with refractory MAC lung disease defined as patients who did not achieve negative sputum cultures after a minimum of 6 consecutive months of a multidrug background regimen therapy. The use of ARIKAYCE is not recommended for patients with non-refractory MAC lung disease.
Managing patients’ expectations
Hear how Dr Wendi Drummond approaches setting patient expectations around potential side effects.
Based on my experience with ARIKAYCE, I think best practices for managing patient expectations includes discussing treatment duration upfront as well as discussing potential side effects and ways that we manage those side effects, should they occur. In my experience, patients are much more likely to remain on the medication if they know what to expect and also know that there are solutions and how we address those potential side effects.
ARIKAYCE is associated with the boxed warning for increased respiratory adverse reactions including hypersensitivity pneumonitis, hemoptysis, bronchospasm, and exacerbation of underlying pulmonary disease that have led to hospitalizations in some cases. Other warnings include ototoxicity, nephrotoxicity, neuromuscular blockade, and embryo fetal toxicity.
It's important to keep in mind that ARIKAYCE is a medication that's added to an already existing multidrug guideline-based therapy regimen and that ARIKAYCE should not be used as monotherapy.
IMPORTANT SAFETY INFORMATION
WARNING: RISK OF INCREASED RESPIRATORY ADVERSE REACTIONS
ARIKAYCE has been associated with an increased risk of respiratory adverse reactions, including hypersensitivity pneumonitis, hemoptysis, bronchospasm, and exacerbation of underlying pulmonary disease that have led to hospitalizations in some cases.
Hypersensitivity Pneumonitis has been reported with the use of ARIKAYCE in the clinical trials. Hypersensitivity pneumonitis (reported as allergic alveolitis, pneumonitis, interstitial lung disease, allergic reaction to ARIKAYCE) was reported at a higher frequency in patients treated with ARIKAYCE plus background regimen (3.1%) compared to patients treated with a background regimen alone (0%). Most patients with hypersensitivity pneumonitis discontinued treatment with ARIKAYCE and received treatment with corticosteroids. If hypersensitivity pneumonitis occurs, discontinue ARIKAYCE and manage patients as medically appropriate.
Hemoptysis has been reported with the use of ARIKAYCE in the clinical trials. Hemoptysis was reported at a higher frequency in patients treated with ARIKAYCE plus background regimen (17.9%) compared to patients treated with a background regimen alone (12.5%). If hemoptysis occurs, manage patients as medically appropriate.
Bronchospasm has been reported with the use of ARIKAYCE in the clinical trials. Bronchospasm (reported as asthma, bronchial hyperreactivity, bronchospasm, dyspnea, dyspnea exertional, prolonged expiration, throat tightness, wheezing) was reported at a higher frequency in patients treated with ARIKAYCE plus background regimen (28.7%) compared to patients treated with a background regimen alone (10.7%). If bronchospasm occurs during the use of ARIKAYCE, treat patients as medically appropriate.
Exacerbations of underlying pulmonary disease has been reported with the use of ARIKAYCE in the clinical trials. Exacerbations of underlying pulmonary disease (reported as chronic obstructive pulmonary disease (COPD), infective exacerbation of COPD, infective exacerbation of bronchiectasis) have been reported at a higher frequency in patients treated with ARIKAYCE plus background regimen (14.8%) compared to patients treated with background regimen alone (9.8%). If exacerbations of underlying pulmonary disease occur during the use of ARIKAYCE, treat patients as medically appropriate.
Anaphylaxis and Hypersensitivity Reactions: Serious and potentially life-threatening hypersensitivity reactions, including anaphylaxis, have been reported in patients taking ARIKAYCE. Signs and symptoms include acute onset of skin and mucosal tissue hypersensitivity reactions (hives, itching, flushing, swollen lips/tongue/uvula), respiratory difficulty (shortness of breath, wheezing, stridor, cough), gastrointestinal symptoms (nausea, vomiting, diarrhea, crampy abdominal pain), and cardiovascular signs and symptoms of anaphylaxis (tachycardia, low blood pressure, syncope, incontinence, dizziness). Before therapy with ARIKAYCE is instituted, evaluate for previous hypersensitivity reactions to aminoglycosides. If anaphylaxis or a hypersensitivity reaction occurs, discontinue ARIKAYCE and institute appropriate supportive measures.
Ototoxicity has been reported with the use of ARIKAYCE in the clinical trials. Ototoxicity (including deafness, dizziness, presyncope, tinnitus, and vertigo) were reported with a higher frequency in patients treated with ARIKAYCE plus background regimen (17%) compared to patients treated with background regimen alone (9.8%). This was primarily driven by tinnitus (7.6% in ARIKAYCE plus background regimen vs 0.9% in the background regimen alone arm) and dizziness (6.3% in ARIKAYCE plus background regimen vs 2.7% in the background regimen alone arm). Closely monitor patients with known or suspected auditory or vestibular dysfunction during treatment with ARIKAYCE. If ototoxicity occurs, manage patients as medically appropriate, including potentially discontinuing ARIKAYCE.
Nephrotoxicity was observed during the clinical trials of ARIKAYCE in patients with MAC lung disease but not at a higher frequency than background regimen alone. Nephrotoxicity has been associated with the aminoglycosides. Close monitoring of patients with known or suspected renal dysfunction may be needed when prescribing ARIKAYCE.
Neuromuscular Blockade: Patients with neuromuscular disorders were not enrolled in ARIKAYCE clinical trials. Patients with known or suspected neuromuscular disorders, such as myasthenia gravis, should be closely monitored since aminoglycosides may aggravate muscle weakness by blocking the release of acetylcholine at neuromuscular junctions.
Embryo-Fetal Toxicity: Aminoglycosides can cause fetal harm when administered to a pregnant woman. Aminoglycosides, including ARIKAYCE, may be associated with total, irreversible, bilateral congenital deafness in pediatric patients exposed in utero. Patients who use ARIKAYCE during pregnancy, or become pregnant while taking ARIKAYCE should be apprised of the potential hazard to the fetus.
Contraindications: ARIKAYCE is contraindicated in patients with known hypersensitivity to any aminoglycoside.
Most Common Adverse Reactions: The most common adverse reactions in Trial 1 at an incidence ≥5% for patients using ARIKAYCE plus background regimen compared to patients treated with background regimen alone were dysphonia (47% vs 1%), cough (39% vs 17%), bronchospasm (29% vs 11%), hemoptysis (18% vs 13%), ototoxicity (17% vs 10%), upper airway irritation (17% vs 2%), musculoskeletal pain (17% vs 8%), fatigue and asthenia (16% vs 10%), exacerbation of underlying pulmonary disease (15% vs 10%), diarrhea (13% vs 5%), nausea (12% vs 4%), pneumonia (10% vs 8%), headache (10% vs 5%), pyrexia (7% vs 5%), vomiting (7% vs 4%), rash (6% vs 2%), decreased weight (6% vs 1%), change in sputum (5% vs 1%), and chest discomfort (5% vs 3%).
Drug Interactions: Avoid concomitant use of ARIKAYCE with medications associated with neurotoxicity, nephrotoxicity, and ototoxicity. Some diuretics can enhance aminoglycoside toxicity by altering aminoglycoside concentrations in serum and tissue. Avoid concomitant use of ARIKAYCE with ethacrynic acid, furosemide, urea, or intravenous mannitol.
Overdosage: Adverse reactions specifically associated with overdose of ARIKAYCE have not been identified. Acute toxicity should be treated with immediate withdrawal of ARIKAYCE, and baseline tests of renal function should be undertaken. Hemodialysis may be helpful in removing amikacin from the body. In all cases of suspected overdosage, physicians should contact the Regional Poison Control Center for information about effective treatment.
INDICATION
LIMITED POPULATION: ARIKAYCE® is indicated in adults, who have limited or no alternative treatment options, for the treatment of Mycobacterium avium complex (MAC) lung disease as part of a combination antibacterial drug regimen in patients who do not achieve negative sputum cultures after a minimum of 6 consecutive months of a multidrug background regimen therapy. As only limited clinical safety and effectiveness data for ARIKAYCE are currently available, reserve ARIKAYCE for use in adults who have limited or no alternative treatment options. This drug is indicated for use in a limited and specific population of patients.
This indication is approved under accelerated approval based on achieving sputum culture conversion (defined as 3 consecutive negative monthly sputum cultures) by Month 6. Clinical benefit has not yet been established. Continued approval for this indication may be contingent upon verification and description of clinical benefit in confirmatory trials.
Limitation of Use: ARIKAYCE has only been studied in patients with refractory MAC lung disease defined as patients who did not achieve negative sputum cultures after a minimum of 6 consecutive months of a multidrug background regimen therapy. The use of ARIKAYCE is not recommended for patients with non-refractory MAC lung disease.
Counseling patients with refractory MAC lung disease
Watch as Dr David Griffith, Dr Nicole Lapinel, and Tracy Drake, RN discuss the value of counseling and management throughout the MAC lung disease journey.
Welcome to Counseling Patients With Refractory MAC (Mycobacterium avium complex) Lung Disease: An ARIKAYCE Case Study.
Today's program will feature a presentation and discussion by our esteemed faculty. Dr David Griffith is a pulmonologist at National Jewish Health. Dr Nicole Lapinel is a pulmonologist at Northwell Health, and Tracy Drake is a registered nurse who manages patients with MAC lung disease at the University of Texas at Tyler.
Thank you all for joining us. Now I'll pass it on to Dr Lapinel to kick off the program.
Thank you.
I would like to start off our discussion by reviewing our objectives. We will discuss perspectives from a multidisciplinary team on counseling patients with refractory MAC lung disease at each step of the management process. We'll also illustrate counseling points by reviewing a real-life refractory MAC lung disease patient case. We will also highlight select ARIKAYCE efficacy and safety data.
MAC lung disease is a chronic condition that requires multiple follow-up visits to evaluate treatment progress. Counseling and a strong partnership between patients and their physicians are fundamental components for the appropriate management of MAC lung disease.
Although the 2020 NTM Treatment Guidelines provide recommendations on how to clinically manage patients with MAC lung disease, patient counseling is noted to be important, but is not necessarily something that's captured for each step of the management process.
Understanding that patient counseling occurs throughout the MAC lung disease journey, we'll be using key milestones based on a real-life patient case as a framework to guide our discussion.
The first step in managing MAC lung disease starts with the initial diagnosis. This visit is crucial because we provide them with a lot of information, and quite honestly it can be very overwhelming for the patients. So, one of the first things that I like to do is to inform my patients that we will be taking a collaborative approach to manage their MAC lung disease. And in this, we will implement a shared decision-making process in order to determine the best course of action.
The reason we implement this approach is to aid patients who can find their diagnosis of MAC lung disease to be quite daunting. Although patients often do their own research and come back with questions, I find that collaborating gives them a voice in how we will manage their disease. The next step that I like to take is to educate my patients about MAC lung disease. And this discussion usually leads into an explanation of the natural history of the disease. With this, I make it a point to counsel my patients on what to expect with the disease. And I tell my patients that studies have found that greater than 50% of patients with MAC lung disease require the initiation of treatment as a result of the disease progression or worsening of their disease over 3 years. I explain this so that they are prepared for the possibility of treatment.
I also like to tell patients this because MAC lung disease is in fact a chronic condition, and they should be aware that if treatment is initiated, it will likely be a very long process that may potentially require years of management.
David, if patients present to you at this step, what else do you discuss?
For most patients, they have little experience or knowledge about nontuberculous mycobacterial (NTM) lung disease. They also have little knowledge about bronchiectasis, which we find almost inevitably in our patients with NTM lung disease. So, I find that the most important part of the initial interaction is education about both bronchiectasis and nontuberculous mycobacterial lung disease.
I tell them that I'm going to make the decision about starting their treatment based on 3 things: their symptoms, what you find in the sputum microbiologically, and their radiographic appearance (what's on their chest x-ray and chest CT [computed tomography] scan).
Frequently, I don't start medication at the first visit with the patient and I like to defer. Again, the first visit is primarily educational and I, I don't like to discuss hypotheticals in terms of what might happen with different disease options. I do explain to them that there are some criteria that we use for starting therapy, earlier than later, including sputum that is AFB (acid-fast bacilli) smear–positive, and patients who have cavitary disease. There is a great deal of discussion, as you know, about the role of watchful waiting in deciding who gets treatment for MAC. And one of the only good things about MAC disease, in general, is that it's a slow process. So you have time to make those decisions, but in a patient with cavitary disease, there is no advantage of course, to watchful waiting.
Thank you, David. So as, as previously discussed in our objectives, we are going to open with, a real-life refractory MAC lung disease case.
So, Mrs CS is a 78-year-old female who is a retired fourth grade teacher who enjoys gardening and lives alone. She has a past medical history of bronchiectasis and used to smoke a half to one pack per day, but she quit at the age of 38 years.
She underwent further evaluation and was diagnosed with MAC lung disease based on persistent cough, shortness of breath, nodular bronchiectasis, and positive AFB sputum cultures.
So, I do find it particularly important to review sputum cultures with patients. One point in particular are the AFB sputum smears. I like to discuss that the AFB sputum smears are risk factor that we do take into consideration when we are trying to decide on treatment initiation, as this can be an important risk factor for progression of disease.
Additionally, given her past history of bronchiectasis, I would emphasize the need for ongoing airway clearance.
Tracy, what do you like to focus on when it comes to airway clearance?
Nicole, I'm glad you brought that up. We, because in our practice, almost all of our patients get some form of airway clearance. We usually start with some OPEP (oscillating positive expiratory pressure) device and then we do the saline and also active cycle breathing. I spend time with the patients going over how to do treatments the proper way, but also how to clean their supplies, their Neb cups and stuff. But then also we go into the order of the therapy from the saline to the OPEP, into the active cycle of breathing. And then I like to discuss with them how to make it part of their daily routine. I get them to understand that it's very important, they either do it one time a day or twice a day,
We are fortunate in our clinic that we actually have a respiratory therapist. So she and I work very hard on trying to make sure our patients understand the importance of airway clearance. We teach the same things. So we start, in terms of breathing techniques, we demonstrate active cycle of breathing. We have them redemonstrate it to us and that's how we end up getting really good sputum specimens.
For patients who have problems with getting secretions out, I always ask them, where do you cough the most? When you lay down flat, do you cough? Do you cough when you're on your side? Or on your stomach? When do you get the most coughing effect? So we go over that quite extensively at how to lay down, how to do postural drainage to get that mucus up and out.
Thank you for that, Tracy. I think that's, very important to talk about airway clearance as a primary form of treatment. And, it sounds like you definitely have a very comprehensive review of the process, inclusive of the respiratory therapist, which is clearly very instrumental in that process.
So as we discussed, many patients will require treatment and will have questions. David, can you share what you discuss with your patients regarding treatment?
After talking to the patients about multidrug therapy and what they can expect, I try to align my expectations of how they would respond to treatment with their own. Typically, I also go into some detail about the medications themselves and the individual toxicity and side effects of each of the medications and how we're going to monitor patients.
I also discuss the importance of microbiologic response to the medication, and specifically, the conversion of their sputum from culture-positive for MAC to culture-negative for MAC. Of course, what's most important to them is are they going to feel better? And so I also discuss how important clinically it is that we see symptomatic improvement and that hopefully we'll see some improvement in lung function.
I also emphasize how important it is for frequent follow-up visits. I think it's important for them and for us. It allows them the opportunity to tell us how they're tolerating the medications, are their symptoms getting better. Are they, not getting better. But most importantly, and this ties in with what Tracy had said about respiratory therapy, it allows us to collect sputum frequently from those patients when they come to the clinic.
So, David, how do you address the need for frequent follow-ups and length of therapy?
I explain to the patients in detail the necessity for the frequent visits, and particularly for frequent collection of sputum for AFB analysis. They have to understand that with the treatment goal of sputum culture negativity, and then a continued 12 months of sputum culture negativity while they're being treated, can only be assessed if we have good sputum specimens routinely through their therapy.
And another reason of course, is for the patients to discuss with us their problems with medications and potential side effects. So, Nicole, how do you describe the side effects of these medications to your patients?
So I like to provide my patients with a high-level overview of some of the most common side effects they may potentially experience when they're on guideline-based therapy. So for example, with macrolides, I frequently will speak about the fact that it can lead to stomach upset. There's also potential for it to cause hearing changes. In the case of rifampin, I might mention that they should not be surprised if some of their bodily fluids might actually turn a different color because that can be something that's quite shocking to patients if, you know, they don't have the readiness for that potential. But also that some of the side effects can even be a little bit more serious, right? So like in the case of ethambutol, it can certainly lead to eye changes. And so for these reasons there's definite necessary follow-up that these patients need to undergo at regular intervals, just to make sure that we don't miss some of the more serious side effects that can occur.
So, I do like to provide my patients with a list of the major side effects that they can experience. I have found that trying to explain, you know, all the possible side effects of any medication can be quite overwhelming for them and they might not necessarily be able to internalize all that.
So, being aware of these common side effects is an integral part of their management plan. So that should they occur that they are, aware that they should get in touch with their healthcare team to make them aware as soon as possible so that we can then again, manage them together.
After patients are told about their side effects, they sometimes ask about the relative risks of starting treatment. So I like to let my patients know that everyone's experience is different and response to treatment can vary. And in my experience, many who start these medications are in fact able to tolerate them. We definitely get a lot of patients that come to us where they have really been told that the treatment is worse than the disease itself, and so we want to make sure that we are preparing them with the knowledge that, that's not necessarily the case, and that some patients can actually go on and can tolerate therapy quite well. And with good clinical response at the end.
So I conclude my discussion with patients on a positive note. And so although studies have shown that two-thirds of patients achieve culture conversion on standard therapy. There are those patients who do not necessarily achieve culture conversion after 6 consecutive months. But I let them know that we do have additional treatment options available. And I like to bring this up early so that my patients are not surprised, if they develop refractory MAC lung disease, as that would be defined. And so it helps prepare them for the possibility of continued treatment with additional options.
So Tracy, after a patient sees their physician, what do you discuss regarding initiating treatment?
This can be a difficult time for patients who are trying to understand MAC lung disease. And as the nurse, I try to provide the patient with multiple handouts so that we can go over everything, but when they go home, they can sit down in their environment and reread everything we've talked about. We provide medication handouts, how to even just take a good nebulizer treatment and how to do it properly, how to clean your equipment, because that's so important. But I also really reinforce what the physician says about getting your eye exams and your hearing tests done and making sure your physicians know that you're on these drugs that can affect your vision or your hearing.
I make sure they know how to reach me, because I want them to be able to reach out to me if they're having side effects so that we can try to negate them and help them through some of those side effects.
I explain the importance of getting your lab work done, go and give them a script that they can take to their local labs and have it done and make sure we get them back. The importance of sending in a sputum every single month, doesn't have to be the same day of every month, but sending in a sputum so that we can track, because that's when we know you're negative, that's when the countdown as they call it starts. So that's very important.
So now I'd like to take this opportunity to return to our patient case. Since Mrs CS had positive AFB cultures, she was started on standard therapy with 3-times-weekly azithromycin 500 milligrams, ethambutol 1200 milligrams, and rifampin 600 milligrams. Some of my patients who have researched standard therapy medications are those who are referred to me, have preconceived notions about treatment. I find that it's important to tell my patients upfront what they can expect with medications. I also try to coach my patients about how to monitor for some of the side effects that they can experience with these medications, as some of them can be quite serious. So I'll provide them with examples of what to watch for and when to notify me or part of my health care team. So for example, I tell patients that if they were to experience a change in their vision, they will likely notice it by not being able to read or see something that they were once able to. And at that point, that would be a good time to reach out to myself or my care team.
David, understanding that patients are typically referred to at this step. Is there anything in particular that you usually share with your patients?
At this step, I have a detailed discussion about the risks and benefits of starting therapy, and if they meet criteria for initiating therapy, and choose not to, that there is evidence that they could experience progressive decline in their lung function and irreversible lung damage.
So after initiating treatment and conducting frequent follow ups, the 2020 NTM Treatment Guidelines recommend evaluating patients at 6 months to assess for treatment response. In my experience, when patients continue to test positive, it can be an emotional time for them. David, how do you support these patients?
Most of the patients that I see are referred at this stage, we unfortunately don't get a lot of people who have not been on therapy before. And I have different approaches based on the patient's reaction to having refractory MAC lung disease.
We had discussed the importance of conversion of sputum to culture-negative at 6 months. So that's the initiation of the process. The patient is aware that at 6 months their sputum is still AFB culture–positive. And we discuss various reasons, for why that might have occurred. We discuss the most dire of those reasons, which is the emergence of macrolide resistance, and we always test the isolates for macrolide susceptibility. So most of the time we know whether or not that's the process, but we again reinforce the idea, are patients taking their medications? How are they taking their medications? Do they do it, according to Tracy's instructions, related to meals or not for instance.
So some patients of course, naturally are going to be, discouraged, disheartened, by the news, but they are usually encouraged by the fact that there are treatment options available, effective treatment options, for their refractory MAC lung disease that we're not going to throw up our hands and say, “Well this is the end of the road.”
So as I had previously discussed with the patient, there is an FDA (US Food and Drug Administration)-approved treatment option for patients with refractory MAC lung disease, which is, amikacin liposome inhalation suspension, or ARIKAYCE. And I talk to them about the data supporting the use of ARIKAYCE in patients with refractory MAC lung disease.
ARIKAYCE is a treatment option for patients with refractory MAC lung disease and is strongly recommended by the 2020 NTM Treatment Guidelines.
When reviewing the clinical data from the CONVERT study, I may not provide my patients with detailed information about the study. However, I find that sharing the information is important for them to understand ARIKAYCE.
The CONVERT study was an open-label, multicenter randomized trial of 336 patients with treatment-refractory MAC lung disease.
Refractory was defined as patients whose sputum remained AFB culture–positive for MAC after 6 months of guideline-based therapy. The patients were randomized 2:1 to either ARIKAYCE plus standard therapy, 224 patients, or standard therapy alone, 112 patients, for a total of up to 16 months.
The primary endpoint was the proportion of patients with culture conversion by Month 6, which was a surrogate endpoint for treatment response. Culture conversion was defined as achieving 3 consecutive monthly negative sputum cultures by Month 6, meaning the first of the 3 negative sputum cultures had to be achieved by Month 4.
Secondary endpoints included change from baseline in 6-minute walk and the St George's Respiratory Questionnaire, culture conversion assessed 12 months after initial culture conversion, and culture conversion assessed 3 months off treatment. The baseline characteristics between the group receiving ARIKAYCE and those not receiving ARIKAYCE were similar, and most patients were older females with underlying structural lung disease, primarily bronchiectasis also including COPD (chronic obstructive pulmonary disease) or both.
The primary endpoint of culture conversion was achieved by significantly more patients on ARIKAYCE plus standard therapy, that was 65 patients or 29% of the group receiving ARIKAYCE compared with patients on standard therapy alone, 10 patients, or 8.9%.
The first culture conversion had to be achieved by Month 4 to meet the primary endpoint of 3 consecutive negative sputum cultures by Month 6. Patients receiving ARIKAYCE plus standard therapy achieved and maintained culture conversion for 12 months and continued to maintain culture conversion for an additional 3 months off treatment. 18.3% of patients receiving ARIKAYCE plus standard therapy maintained culture conversion 12 months after their first culture conversion compared with 2.7% of patients on standard therapy alone.
Furthermore, 16.1% of patients receiving ARIKAYCE plus standard therapy continued to maintain culture conversion 3 months off treatment compared with no patients on standard therapy alone.
By Month 6, the change from baseline in 6-minute walk test and St George's Respiratory Questionnaire did not demonstrate clinical benefit.
The most common adverse reactions observed in the CONVERT study were respiratory in nature and generally mild to moderate in severity.
Dysphonia, cough, bronchospasm, and hemoptysis were some of the most common adverse reactions reported in patients who received ARIKAYCE plus standard therapy.
Treatment-emergent adverse events (TEAEs) reported in more than 10% of patients who received ARIKAYCE plus standard therapy included dysphonia, cough, hemoptysis, dyspnea, fatigue, diarrhea, nausea, and oropharyngeal pain. Most of the common treatment emergent adverse events occurred more frequently in patients who received ARIKAYCE plus standard therapy, except hemoptysis, which was similar across both treatment arms. As shown in the graph for ARIKAYCE plus standard therapy, the emergence of most TEAEs were reported within the first month with declining incidence of reports of new onset thereafter.
It is important to note that the graphs show the month the TEAEs were first reported after initiation of therapy, and that there are no data on the resolutions of these TEAEs. That is the incidence of TEAEs does not decrease because of resolution. Discontinuation due to TEAEs primarily occurred early in the trial and declined over time.
Another aspect I like to bring to my patient's attention is how the targeted delivery of ARIKAYCE into the lungs allows for less systemic exposure to amikacin.
I tell my patients that the CONVERT trial enrolled patients who, like them, had refractory MAC lung disease. I explain to my patients that nearly one-third of patients who received ARIKAYCE plus standard therapy achieved culture conversion by Month 6, and more than half of those patients stayed culture converted. The reason I provide my patients with a brief summary of the data is because I like to help them feel confident in their treatment decision.
Just like how Nicole provides patients with high-level overview of the major side effects that can be expected when initiating standard therapy, I counsel patients on what side effects to expect with ARIKAYCE. I note that the most common side effects were dysphonia, cough, bronchospasm, and hemoptysis. Additionally, I have found it imperative to let patients know that about 50% of patients in the trial experience dysphonia, because it can be an unsettling side effect if they are not made aware. I educate patients that the most common side effects were respiratory in nature and mild to moderate and severity. And most patients who experienced the side effect in the trial did not stop treatment. I also inform my patients that the emergence of most TEAES were reported within the first month, with declining incidence of reports of new onset thereafter.
Thank you, David, for walking us through the data and highlighting some of the key points that you communicate to your patients when starting ARIKAYCE.
Our patient Mrs CS was initiated on ARIKAYCE. Her AFB sputum cultures remained positive, and she continued to grow Mycobacterium intracellulare after 6 months of standard therapy. She was told to expect that treatment would continue for months after she achieved culture conversion. It's at this stage I would tell my patients that ARIKAYCE is an inhaled medication that requires its own specific nebulizer, called the Lamira® Nebulizer System. I would reiterate that we will obtain baseline audiograms and continue to monitor for treatment response and potential side effects. Similar to how Mrs CS was reminded about the long-term treatment journey associated with MAC lung disease, I would tell my patients to expect to be on treatment until well after achieving culture conversion per the 2020 NTM Treatment Guidelines.
So, Tracy, what information and support do you provide to your patients when starting them on ARIKAYCE in addition to standard therapy?
A lot of our patients think that when a medicine is ordered it goes to the pharmacy and they will get it the next day. But with ARIKAYCE, we have to let them know that there's a process and that it has to go to a specialty pharmacy.
And so I provide them with the handout that the Arikares support group gives us. It lets the patient know about their journey in these little handouts. And then I also inform them that the specialty pharmacy will have to talk to them before they will ever mail the package. They've got to make sure someone's going to be there for delivery.
And understanding that ARIKAYCE has its own nebulizer system and that when the patient enrolls in the support group or the Arikares support program, then they actually have a trainer that will come out and help them. But also Arikares supports them through the treatment journey, including voluntary training, but also that trainer is there if they have other questions too.
For patients who use the bronchodilator, I actually talk to them about what the bronchodilator does, what's it doing in the airways. Then I talk about how the saline works, and then what each part of the OPEP and the active cycle of breathing, how that's working to get the mucus out. So, by the time that we've done all this talking, the nebulized treatment or the inhaler treatment for the bronchodilator should already be in effect, because it takes about 14 to 20 minutes.
And I like to spend time with them saying, you know, this is your daily routine. This is going to be every day, you need to figure out what's the easiest way for you to get it done and how to get it done, but to understand that it takes time out of their day to get the ARIKAYCE done, but to do it. I also like to reiterate about the side effects that Dr Griffith referred to and encourage patients to reach out to the clinic when they start experiencing any side effects or have any other questions.
So knowing that most of the patients in the trial experienced a side effect while receiving ARIKAYCE, David, what do you tell your patients about the management of side effects after starting ARIKAYCE?
The most important thing is for patients to know that they can communicate with me or someone on our team about the problems that they're having. Now, depending on the severity of the problem, I, I look at the side effects associated with ARIKAYCE as I would the side effects of any other medicine.
How do you address the management of specific side effects?
We discuss various strategies that we can implement to manage the side effects. I let them know that when the time comes that we will discuss those specific strategies. And I find that the more communication that we have, patients are better prepared when they experience the onset of one of these side effects that it's not such a disruptive process.
So, Tracy, what additional support would you provide to patients at this step?
Depending on our management strategy, I would be sure to let them know the order and timing of how to take the therapies and the supported therapy, the treatments. For example, if they're on saline, the hypertonic, then I discuss with them when they should be using the nebulizer, how to do things in order. If the patient are asked to start albuterol, and again, I reiterate teaching. Make sure they do a proper administration if they're doing an MDI (metered dose inhaler), but if they're doing a nebulizer, are they breathing correctly? Just to make sure that we're getting everything the best of everything. I found that it's best to give patients a few tips at a time, so they don't feel overwhelmed. So when they call, then I give them more information, but you want to just give them basic stuff at first.
After a patient has informed me of a side effect they're experiencing, I will use my clinical judgment to decide which intervention is best. I like to reference a postmarketing telephone survey I participated in of 26 patients who were prescribed ARIKAYCE that was conducted during a 2-month period at 2 academic medical centers in the United States. So again, because each patient responds to treatment differently, the same intervention may not work for everyone. In this survey, we evaluated patients with side effects related to increased coughing, dysphonia, dyspnea, and increased sputum. And as you can see on the slide, there are various interventions that were undertaken.
Important to note this information is not included in the ARIKAYCE full Prescribing Information. Writing assistance for the study was provided to the authors through funding from Insmed incorporated. Insmed was not involved with the conceptualization, development, conduct, or analyses of the survey. And the phone survey identifies strategies that were used by some patients for some of the more common side effects.
So Tracy, how do you like to discuss side effects with your patients?
Similar to what was reported in the survey, if my patients experience dysphonia then I talk to them about the importance of gargling with warm water. For patients that are feeling short of breath or coughing, bronchospasms, then I'll talk to them about using the bronchodilator.
Within a few days of starting ARIKAYCE Mrs CS developed worsening cough and increased sputum production. Based on these symptoms, Mrs CS was instructed to temporarily stop ARIKAYCE and nebulization with 7% sodium chloride. She was also informed that she could resume ARIKAYCE nebulization with 3% hypertonic saline once her symptoms had subsided. It is important to medically manage patients on a case-by-case basis. So in addition to her action plan, Mrs CS was provided with clear direction to use her albuterol prior to taking ARIKAYCE and to avoid nebulizing within four hours of taking ARIKAYCE. At this step, Mrs CS was counseled to repeat the same process if she was to experience these side effects again.
Tracy, how do you help reinforce the action plan for side effect management?
As I'm typically the first point of contact when patients call, I make sure that I'm frequently are being updated by the physicians regarding the side effects that the patients may be having. And we develop management plan. This way when the patient calls, if they're experiencing the same side effects, then I can pull up the previous action plan, go back through it, walk them through it. And again, bringing it back to the physician's attention if it's needed. If a patient calls with a different side effect, then again, I triage the patient by asking the standard questions around the order of therapy. I also use it as an opportunity to discuss various strategies to help manage the side effects, noting that the approach should be individualized to each patient.
Tracy, how do you support patients during this time?
Experiencing side effects can be hard on patients. And so I try to be very supportive of other obstacles that they're going through. So I want to be there for them and let them know that, "Hey, we can, you can do this". I make sure they understand how to reach me during the working hours and then who they can call after hours. I also try to provide them with resources, support groups, so they can read up on the discussion about MAC and NTM and this disease. And I always end our phone call conversations with, “Did I answer all your questions?”, and make sure that they don't have anything just hanging out there, that they still don't understand.
David, how do you describe what follow-ups will entail to patients who continue treatment with ARIKAYCE?
Well, first, I reassure them of our commitment to completing therapy and to be there for them through the remainder of their journey. But second, I emphasize to them that the same questions that we have during the initial part of the therapy are still pertinent. And so we want to know how they're responding to therapy, and we want to know if they're having side effects with their therapy. So I emphasize to them that the monitoring is no less intense than it was prior to beginning ARIKAYCE.
Despite having experienced side effects, Mrs CS was able to resume treatment with ARIKAYCE and tolerate therapy without the recurrence of any side effects. As previously mentioned, everyone's experience with ARIKAYCE will be different. At her next follow-up, her cultures were negative for Mycobacterium intracellulare and her AFB sputum cultures were negative.
In summary, I would like to review some key counseling points for consideration at each step of the management process for MAC lung disease.
At diagnosis, it's extremely important that we review MAC lung disease education, as well as emphasize that this is a shared decision-making process between the patient and the physician. At the time of initiation of standard therapy, there are several important points that need to be reviewed. One, it's important to review the goals of treatment. Two, to ready patients of the need for frequent follow-ups. Three, it is important to review the potential side effects that can occur with treatment with guideline-based therapy. Four, it's also important to make patients aware of the possibility of refractory MAC lung disease, which is patients that have been on guideline-based therapy who have not achieved culture conversion by 6 months. And lastly, the fact that there are alternative treatment options available at the 6-month reassessment and adding ARIKAYCE.
So it's important to provide patients with education about refractory MAC lung disease. With this, it's important to review the CONVERT trial data and the potential side effects that can be associated with ARIKAYCE.
Lastly, it's important to educate patients on the side effect profile associated with ARIKAYCE. It's important that patients are aware of what potential side effects they can experience with ARIKAYCE, but also that there are potential management strategies available to manage these side effects.
Thank you for viewing this webinar. We hope you found the content helpful. We also want to thank our faculty for their time and expertise in delivering this program. Also, if you haven't already, please feel free to explore the ARIKAYCE Academy. The ARIKAYCE Academy is a new digital platform that provides information about ARIKAYCE and refractory MAC lung disease. We are excited to share this new platform with you. Thank you again.
ARIKAYCE should be used in the MAC lung disease patient who is not responding, or who's not responded, to a standard multidrug regimen for MAC. These are patients who have been on treatment for at least 6 months and remain culture-positive.
IMPORTANT SAFETY INFORMATION
WARNING: RISK OF INCREASED RESPIRATORY ADVERSE REACTIONS
ARIKAYCE has been associated with an increased risk of respiratory adverse reactions, including hypersensitivity pneumonitis, hemoptysis, bronchospasm, and exacerbation of underlying pulmonary disease that have led to hospitalizations in some cases.
Hypersensitivity Pneumonitis has been reported with the use of ARIKAYCE in the clinical trials. Hypersensitivity pneumonitis (reported as allergic alveolitis, pneumonitis, interstitial lung disease, allergic reaction to ARIKAYCE) was reported at a higher frequency in patients treated with ARIKAYCE plus background regimen (3.1%) compared to patients treated with a background regimen alone (0%). Most patients with hypersensitivity pneumonitis discontinued treatment with ARIKAYCE and received treatment with corticosteroids. If hypersensitivity pneumonitis occurs, discontinue ARIKAYCE and manage patients as medically appropriate.
Hemoptysis has been reported with the use of ARIKAYCE in the clinical trials. Hemoptysis was reported at a higher frequency in patients treated with ARIKAYCE plus background regimen (17.9%) compared to patients treated with a background regimen alone (12.5%). If hemoptysis occurs, manage patients as medically appropriate.
Bronchospasm has been reported with the use of ARIKAYCE in the clinical trials. Bronchospasm (reported as asthma, bronchial hyperreactivity, bronchospasm, dyspnea, dyspnea exertional, prolonged expiration, throat tightness, wheezing) was reported at a higher frequency in patients treated with ARIKAYCE plus background regimen (28.7%) compared to patients treated with a background regimen alone (10.7%). If bronchospasm occurs during the use of ARIKAYCE, treat patients as medically appropriate.
Exacerbations of underlying pulmonary disease has been reported with the use of ARIKAYCE in the clinical trials. Exacerbations of underlying pulmonary disease (reported as chronic obstructive pulmonary disease (COPD), infective exacerbation of COPD, infective exacerbation of bronchiectasis) have been reported at a higher frequency in patients treated with ARIKAYCE plus background regimen (14.8%) compared to patients treated with background regimen alone (9.8%). If exacerbations of underlying pulmonary disease occur during the use of ARIKAYCE, treat patients as medically appropriate.
Anaphylaxis and Hypersensitivity Reactions: Serious and potentially life-threatening hypersensitivity reactions, including anaphylaxis, have been reported in patients taking ARIKAYCE. Signs and symptoms include acute onset of skin and mucosal tissue hypersensitivity reactions (hives, itching, flushing, swollen lips/tongue/uvula), respiratory difficulty (shortness of breath, wheezing, stridor, cough), gastrointestinal symptoms (nausea, vomiting, diarrhea, crampy abdominal pain), and cardiovascular signs and symptoms of anaphylaxis (tachycardia, low blood pressure, syncope, incontinence, dizziness). Before therapy with ARIKAYCE is instituted, evaluate for previous hypersensitivity reactions to aminoglycosides. If anaphylaxis or a hypersensitivity reaction occurs, discontinue ARIKAYCE and institute appropriate supportive measures.
Ototoxicity has been reported with the use of ARIKAYCE in the clinical trials. Ototoxicity (including deafness, dizziness, presyncope, tinnitus, and vertigo) were reported with a higher frequency in patients treated with ARIKAYCE plus background regimen (17%) compared to patients treated with background regimen alone (9.8%). This was primarily driven by tinnitus (7.6% in ARIKAYCE plus background regimen vs 0.9% in the background regimen alone arm) and dizziness (6.3% in ARIKAYCE plus background regimen vs 2.7% in the background regimen alone arm). Closely monitor patients with known or suspected auditory or vestibular dysfunction during treatment with ARIKAYCE. If ototoxicity occurs, manage patients as medically appropriate, including potentially discontinuing ARIKAYCE.
Nephrotoxicity was observed during the clinical trials of ARIKAYCE in patients with MAC lung disease but not at a higher frequency than background regimen alone. Nephrotoxicity has been associated with the aminoglycosides. Close monitoring of patients with known or suspected renal dysfunction may be needed when prescribing ARIKAYCE.
Neuromuscular Blockade: Patients with neuromuscular disorders were not enrolled in ARIKAYCE clinical trials. Patients with known or suspected neuromuscular disorders, such as myasthenia gravis, should be closely monitored since aminoglycosides may aggravate muscle weakness by blocking the release of acetylcholine at neuromuscular junctions.
Embryo-Fetal Toxicity: Aminoglycosides can cause fetal harm when administered to a pregnant woman. Aminoglycosides, including ARIKAYCE, may be associated with total, irreversible, bilateral congenital deafness in pediatric patients exposed in utero. Patients who use ARIKAYCE during pregnancy, or become pregnant while taking ARIKAYCE should be apprised of the potential hazard to the fetus.
Contraindications: ARIKAYCE is contraindicated in patients with known hypersensitivity to any aminoglycoside.
Most Common Adverse Reactions: The most common adverse reactions in Trial 1 at an incidence ≥5% for patients using ARIKAYCE plus background regimen compared to patients treated with background regimen alone were dysphonia (47% vs 1%), cough (39% vs 17%), bronchospasm (29% vs 11%), hemoptysis (18% vs 13%), ototoxicity (17% vs 10%), upper airway irritation (17% vs 2%), musculoskeletal pain (17% vs 8%), fatigue and asthenia (16% vs 10%), exacerbation of underlying pulmonary disease (15% vs 10%), diarrhea (13% vs 5%), nausea (12% vs 4%), pneumonia (10% vs 8%), headache (10% vs 5%), pyrexia (7% vs 5%), vomiting (7% vs 4%), rash (6% vs 2%), decreased weight (6% vs 1%), change in sputum (5% vs 1%), and chest discomfort (5% vs 3%).
Drug Interactions: Avoid concomitant use of ARIKAYCE with medications associated with neurotoxicity, nephrotoxicity, and ototoxicity. Some diuretics can enhance aminoglycoside toxicity by altering aminoglycoside concentrations in serum and tissue. Avoid concomitant use of ARIKAYCE with ethacrynic acid, furosemide, urea, or intravenous mannitol.
Overdosage: Adverse reactions specifically associated with overdose of ARIKAYCE have not been identified. Acute toxicity should be treated with immediate withdrawal of ARIKAYCE, and baseline tests of renal function should be undertaken. Hemodialysis may be helpful in removing amikacin from the body. In all cases of suspected overdosage, physicians should contact the Regional Poison Control Center for information about effective treatment.
INDICATION
LIMITED POPULATION: ARIKAYCE® is indicated in adults, who have limited or no alternative treatment options, for the treatment of Mycobacterium avium complex (MAC) lung disease as part of a combination antibacterial drug regimen in patients who do not achieve negative sputum cultures after a minimum of 6 consecutive months of a multidrug background regimen therapy. As only limited clinical safety and effectiveness data for ARIKAYCE are currently available, reserve ARIKAYCE for use in adults who have limited or no alternative treatment options. This drug is indicated for use in a limited and specific population of patients.
This indication is approved under accelerated approval based on achieving sputum culture conversion (defined as 3 consecutive negative monthly sputum cultures) by Month 6. Clinical benefit has not yet been established. Continued approval for this indication may be contingent upon verification and description of clinical benefit in confirmatory trials.
Limitation of Use: ARIKAYCE has only been studied in patients with refractory MAC lung disease defined as patients who did not achieve negative sputum cultures after a minimum of 6 consecutive months of a multidrug background regimen therapy. The use of ARIKAYCE is not recommended for patients with non-refractory MAC lung disease.
Review expert commentary on topics related to the management of MAC lung disease


Julie Philley, MD, Pulmonary and Critical Care Medicine Expert
What is the significance of the 6-month mark while treating with multidrug therapy? In this article, Dr Julie Philley reviews steps for what's next when patients do not experience culture conversion.
Frequent monitoring is vital for successful long-term patient management.1 The 6-month evaluation uncovers whether or not a patient is responding to initial treatment early in the disease course,1 and patients are considered to have refractory MAC lung disease if they do not culture convert after 6 months.2 The lack of culture conversion by 6 months may be predictive of treatment failure at 12 months.3 This challenge is important to address because there is a risk of greater decline in lung function4,5 and worse outcomes in patients who are not responding to standard therapy.6 I check sputums monthly, and if culture conversion is not achieved by 6 months, many of my patients require additional treatment. The next step in my management strategy is to expand the therapeutic regimen based on the 2020 ATS/ERS/ESCMID/IDSA NTM Treatment Guidelines. Timely initiation of therapy after 6 months of failure to convert is essential to creating an optimal treatment journey.1
Watch experts discuss how they counsel patients through the 6-month treatment milestone >
Watch experts discuss how they counsel patients through the 6-month treatment milestone >
ATS, American Thoracic Society; ERS, European Respiratory Society; ESCMID, European Society of Clinical Microbiology and Infectious Diseases; IDSA, Infectious Diseases Society of America; MAC, Mycobacterium avium complex; NTM, nontuberculous mycobacteria.
Dr Philley was compensated for her time in creating this material.
References: 1. Daley CL, Iaccarino JM Jr, Lange C, et al. Treatment of nontuberculous mycobacterial pulmonary disease: an official ATS/ERS/ESCMID/IDSA Clinical Practice Guideline. Clin Infect Dis. 2020;71(4):e1-e36. doi:10.1093/cid/ciaa241 2. ARIKAYCE. Prescribing information. Insmed Incorporated; October 2020 3. Moon SM, Jhun BW, Daley CL, Koh W-J. Unresolved issues in treatment outcome definitions for nontuberculous mycobacterial pulmonary disease. Eur Respir J. 2019;53(5):1801636. doi:10.1183/13993003.01636-2018 4. Park HY, Jeong B-H, Chon HR, Jeon K, Daley CL, Koh W-J. Lung function decline according to clinical course in nontuberculous mycobacterial lung disease. Chest. 2016;150(6):1222-1232. doi:10.1016/j.chest.2016.06.005 5. Huang C-T, Tsai Y-J, Wu H-D, et al. Impact of non-tuberculous mycobacteria on pulmonary function decline in chronic obstructive pulmonary disease. Int J Tuberc Lung Dis. 2012;16(4):539-545. doi:10.5588/ijtld.11.0412 6. O’Connell ML, Birkenkamp KE, Kleiner DE, Folio LR, Holland SM, Olivier KN. Lung manifestations in an autopsy-based series of pulmonary or disseminated nontuberculous mycobacterial disease. Chest. 2012;141(5):1203-1209. doi:10.1378/chest.11-0425
PP-ARIK-US-01683


Pamela McShane, MD, Pulmonary and Critical Care Medicine Expert
Now that you've made the decision to add ARIKAYCE, what should you expect? This article by Dr Pamela McShane summarizes goals and recommendations for management, as well as endpoints and safety results from the CONVERT study.
Culture conversion is an important goal of therapy for my patients with MAC lung disease because it is an objective measure of the status of the infection.1 Taking into consideration the individual patient’s presentation, I believe it makes sense to adjust treatment after Month 6 in patients whose cultures have not converted. Some patients with refractory MAC lung disease may have limited treatment options; however, there is an effective treatment that has been shown to improve their odds of achieving culture conversion by Month 12 and beyond.2
At the 6‐month critical time point, the 2020 ATS/ERS/ESCMID/IDSA NTM Treatment Guidelines strongly recommend adding ARIKAYCE® (amikacin liposome inhalation suspension),3 which has demonstrated efficacy in patients with refractory MAC lung disease.2
The clinical data supporting ARIKAYCE comes from the Phase 3 CONVERT (INS 212) study.
- Open‐label, multicenter, randomized trial that included 336 patients with refractory MAC lung disease2,4 (224 patients received ARIKAYCE plus standard therapy and 112 patients received standard therapy alone)2
- Primary endpoint: proportion of patients who culture converted by Month 62
- ARIKAYCE plus standard therapy (n=65 [29.0%]) compared with standard therapy alone (n=10 [8.9%]) (P<0.0001)
- Secondary endpoints2
- Change in 6MWT and SGRQ scores at Month 6
- ARIKAYCE did not demonstrate clinical benefit
- Remained culture‐negative for 12 months of treatment
- 18.3% of patients who received ARIKAYCE plus standard therapy compared with 2.7% of patients who received standard therapy alone (P<0.0001)
- Remained culture‐negative for 12 months of treatment and 3 months off treatment (ITT population)
- 16.1% of patients who received ARIKAYCE plus standard therapy compared with 0% of patients who received standard therapy alone
- Change in 6MWT and SGRQ scores at Month 6
- Safety
- The most common adverse reactions were respiratory in nature and mild to moderate in severity5
- Dysphonia, cough, bronchospasm, and hemoptysis were the most common adverse reactions reported in patients who received ARIKAYCE plus standard therapy2
The data from the CONVERT study suggest that ARIKAYCE helps achieve and maintain culture conversion rates, with a greater number of patients remaining culture‐negative for 12 months. More than half of patients who achieved culture conversion with ARIKAYCE maintained conversion for 3 months off therapy, whereas no patients receiving standard therapy alone maintained culture conversion for the same amount of time. In the CONVERT study, standard therapy alone was not shown to help patients with refractory MAC lung disease stay culture-negative. ARIKAYCE has been shown to help patients achieve sustained culture conversion that lasts months after discontinuation of therapy.2 With these data in mind, I feel confident when adding ARIKAYCE to my patients’ treatment regimens.
6MWT, 6‐minute walk test; ATS, American Thoracic Society; ERS, European Respiratory Society; ESCMID, European Society of Clinical Microbiology and Infectious Diseases; IDSA, Infectious Diseases Society of America; ITT, intent‐to‐treat; MAC, Mycobacterium avium complex; NTM, nontuberculous mycobacteria; SGRQ, St George’s Respiratory Questionnaire.
Dr McShane was compensated for her time in creating this material.
References: 1. Griffith DE, Adjemian J, Brown‐Elliott BA, et al. Semiquantitative Culture Analysis During Therapy for Mycobacterium avium Complex Lung Disease. Am J Respir Crit Care Med. 2015;192(6):754‐760. doi:10.1164/rccm.201503‐0444OC 2. ARIKAYCE Prescribing information. Insmed Incorporated; October 2020 3. Daley CL, Iaccarino JM Jr, Lange C, et al. Treatment of nontuberculous mycobacterial pulmonary disease: an official ATS/ERS/ESCMID/IDSA Clinical Practice Guideline. Clin Infect Dis. 2020;71(4):e1‐e36. doi:10.1093/cid/ciaa241 4. Griffith DE, Eagle G, Thomson R, et al; for the CONVERT Study Group. Amikacin liposome inhalation suspension for refractory Mycobacterium avium complex lung disease: sustainability and durability of culture conversion and safety of long‐term exposure. Chest. 2021;160(3):831‐842. doi:10.1016/j.chest.2021.03.070 5. Griffith DE, Eagle G, Thomson R, et al; for the CONVERT Study Group. Amikacin liposome inhalation suspension for treatment‐refractory lung disease caused by Mycobacterium avium complex (CONVERT): a prospective, open‐label, randomized study. Am J Respir Crit Care Med. 2018;198(12):1559‐1569. doi:10.1164/rccm.201807‐1318OC
PP‐ARIK‐US‐01684

Nicole Lapinel, MD
Patient counseling is critical at diagnosis and throughout the treatment journey. In this advertorial, Dr Nicole Lapinel shares her approach to counseling patients with MAC lung disease.
 Download now
Download now 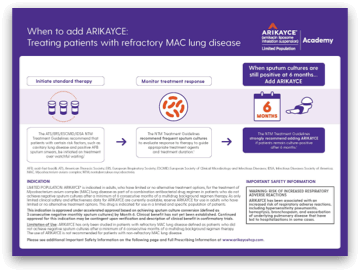
This convenient piece provides guidelines for managing MAC lung disease and adding ARIKAYCE in a handy, at-a-glance format.
 Download now
Download now NTM=nontuberculous mycobacteria.